This article reviews the best evidence available to guide the follow-up of patients with melanoma, focusing on incidence of, and detection of, melanoma recurrence, frequency of follow-up visits, yield of, laboratory and radiographic tests, outcomes of patients with recurrent melanoma based on method of detection, detection of secondary melanomas, and stage-specific follow-up.
When a patient with a solid tumor has been found to have no evidence of disease (NED) by a combination of surgery, chemotherapy, and/or radiation, they are entered into a follow-up program that entails a combination of clinical visits with history and physical examination, laboratory and radiographic investigations, and psychosocial support. The frequency and intensity of visits and choice of studies are generally based on the timing and patterns of recurrence for that particular malignancy. The primary goal of a follow-up program is early detection of recurrent disease, and is based on the premise that earlier detection of tumor recurrence allows for treatment and potential cure of recurrent disease. A secondary goal of a follow-up program is detection of second primary tumors, which is based on the observation in many solid malignancies that patients are at higher risk for developing secondary malignancies compared with the general population. Tertiary goals of follow-up programs include psychosocial support for the surviving patient and systematic recording of outcomes to track the efficacy of treatment regimens.
Conceptually, if early detection of recurrence and/or new primary lesions can lead to improved survival, the surface location, locoregional recurrence patterns, and increased rates of secondary tumors make melanoma ideal for a program of intensive follow-up. Overall, among patients whose melanoma recurs, 20% to 28% first recur with local or in-transit disease, 26% to 60% with regional nodal disease, and 15% to 50% with distant metastases. When melanoma recurs locally, in-transit, or in regional nodal basins, approximately one-third of patients can be cured with additional treatment. In contrast, patients with distant metastases have a more dismal prognosis, with 5-year survival less than 10%. However, there is a small, highly selected subset of patients with resectable stage IV tumors who can achieve 5-year survival of 20%.
On the one hand, a physician who treats patients with melanoma could argue that intensive follow-up identifies patients with early recurrences who could be effectively treated to positively affect survival. On the other hand, with decades of experience with intensive follow-up programs, there is no level 1 evidence (and scant level 2–4 evidence) to support the hypothesis that more intensive follow-up of the patient with melanoma (consisting of combinations of history, physical examination, laboratory tests, and radiographic studies) has any effect on survival. Furthermore, both the rising prevalence of melanoma and the fact that most patients with melanoma are rendered NED after initial treatment, have led to more patients who need to be enrolled in follow-up programs for longer periods, creating a scenario in which providers of melanoma care could be overwhelmed with cured patients in follow-up programs. Even the basic premise of early detection of recurrence leading to improved outcomes is questionable, because there is no effective treatment that leads to improved survival in patients with recurrent systemic melanoma. All of these factors, along with the need for cost-effective health care, have led many to question the need for and usefulness of intensive follow-up in patients with melanoma and to stress the importance of better prospective studies to evaluate the benefit of follow-up.
There are no universally accepted guidelines for the follow-up of patients with melanoma. Most follow-up schedules are based on timing and patterns of recurrence derived from large retrospective reviews. Patient-related features, such as the definition of a patient-detected recurrence, clinician-detected recurrence, and the breakdown of symptomatic and asymptomatic recurrences and their corresponding definitions, limits comparisons of these studies. There is significant variability in follow-up recommendations from various organizations ( Table 1 ) across all continents. The efficacy and outcomes of these follow-up programs are also retrospective in nature. Thus, the evidence for and against various types of follow-up is weak (level 3 and lower). Only one prospective study in the literature addresses melanoma follow-up, and this was methodologically limited by the inclusion of patients with known metastatic disease. To date, the most methodologically complete data on follow-up of patients with melanoma come from 2 comprehensive literature reviews.
| Follow-up Category | Consensus Group Recommendations | ||
|---|---|---|---|
| NCCN (United States) | Australia/New Zealand | Germany | |
| Self-examination Recommended? | Yes | Yes | Not stated |
| Follow-up Interval | |||
| Stage I | 3–12 mo × 5 years a | 6 mo × 5 years b | 6 mo × 5 years c |
| Stage II and III | 3 – 6 mo × 2 years a 3–12 months × 3 years a | 3–4 mo × 5 years b | 3 mo × 5 years d |
| Routine Imaging? | |||
| Stage I | No | No | No |
| Stage II and III | Consider e | No | Yes f |
| Routine Blood Work? | No | No | Yes g |
a Annual follow-up after 5 years as clinically indicated.
b Annual follow-up after 5 years.
c 6- to 12-month follow-up in years 6 to 10.
d 6-month follow-up in years 6 to 10.
e Consider CXR, CT, and/or PET-CT scans to screen for recurrent/metastatic disease. Consider brain MRI annually. Routine imaging is not recommended after 5 years.
f Regional lymph node US for stage II (every 6 months) and III (every 3 months) melanomas for 5 years. Abdominal US and CXR or CT, MRI, or PET scan at each visit for 5 years.
g Serum S-100B protein levels every 3 to 6 months for stage II and III melanomas.
This article reviews the best evidence available to guide the follow-up of patients with melanoma, focusing on incidence and detection of melanoma recurrence, frequency of follow-up visits, yield of laboratory and radiographic tests, outcomes of patients with recurrent melanoma based on method of detection, detection of secondary melanomas, and stage-specific follow-up.
Incidence of melanoma recurrence during follow-up
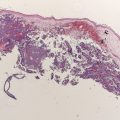
Of all patients with stage I to III completely resected melanomas, 30% develop a recurrence, and approximately 80% of these occur within 3 years of diagnosis of their primary melanoma. Although it is rare for a melanoma to recur after 10 years of follow-up, there are case reports of recurrent melanoma up to 46 years after treatment of a primary melanoma. Late recurrence of melanoma (>10 years after treatment) is largely an observation limited to patients who initially present with early, node-negative primary tumors.
The probability of recurrence is clearly stage dependent. In an analysis of 4748 patients with stage I and II melanoma, melanoma recurred in 18.9% of patients: 5.2% for stage IA, 18.4% for stage IB, 28.7% for stage IIA, 40.6% for stage IIB, and 44.3% for stage IIC.
Patterns of melanoma recurrence during follow-up
In their comprehensive literature review in 2005, Francken and colleagues identified 8 studies that reported the incidence of recurrence in patients with melanoma in follow-up programs. In all patients, most of whom were stage I or II, the rates of recurrence were 3% to 5% for local or in-transit, 5% to 13% for regional nodal, and 3% to 10% for distant recurrence. Among patients whose melanoma recurred, 20% to 28% first present with local or in-transit recurrences, 26% to 60% with regional nodal recurrences, and 15% to 50% with distant recurrences. The wide variation in recurrence rates and patterns can likely be explained by the different proportion of stage I, II, and III patients, as well as how they were staged in these studies. For example, Baughan and colleagues included only stage I patients in their analysis, whereas Hoffman and colleagues included all completely resected stage I, II, and III patients in their series. Most recently, Francken and colleagues from the Sydney Melanoma Unit showed that the site of first recurrence in 211 patients with stage I to III melanoma was local in 13% of patients, in-transit in 17%, regional in 46%, and distant in 24% of patients.
Patterns of melanoma recurrence during follow-up
In their comprehensive literature review in 2005, Francken and colleagues identified 8 studies that reported the incidence of recurrence in patients with melanoma in follow-up programs. In all patients, most of whom were stage I or II, the rates of recurrence were 3% to 5% for local or in-transit, 5% to 13% for regional nodal, and 3% to 10% for distant recurrence. Among patients whose melanoma recurred, 20% to 28% first present with local or in-transit recurrences, 26% to 60% with regional nodal recurrences, and 15% to 50% with distant recurrences. The wide variation in recurrence rates and patterns can likely be explained by the different proportion of stage I, II, and III patients, as well as how they were staged in these studies. For example, Baughan and colleagues included only stage I patients in their analysis, whereas Hoffman and colleagues included all completely resected stage I, II, and III patients in their series. Most recently, Francken and colleagues from the Sydney Melanoma Unit showed that the site of first recurrence in 211 patients with stage I to III melanoma was local in 13% of patients, in-transit in 17%, regional in 46%, and distant in 24% of patients.
Detection of melanoma recurrence
In general, melanoma recurrences are detected more often by patients than by health care professionals at scheduled follow-up visits. Numerous reports in the literature indicate that three-quarters of all recurrences are detected by patients, whereas one-quarter are detected by clinicians. However, some series have found the opposite, with increased rates of recurrences detected by health care professionals. Overall, the rate of first recurrences detected by patients from all reports in the literature is 33% to 99%. Even in stage III patients, who could be considered at highest risk for systemic recurrence and might benefit most from physician-directed follow-up examination and studies, 62% of local and in-transit recurrences, 49% of nodal recurrences, and 37% of systemic recurrences were initially detected by the patients. The retrospective nature of these reports and varied methods of detection of recurrence make comparisons difficult. For example, some studies used questionnaires that were mailed out to all patients whose melanoma recurred and asked them to recall who detected the recurrence, whereas other studies audited the medical record to determine who detected the first recurrence. Yet others based definition of a patient-detected recurrence as one found on an unscheduled clinic visit. In the single prospective study evaluating follow-up of patients with melanoma, only 17% of recurrences were detected by the patients. However, this study captured all recurrences (first and subsequent), so it is difficult to compare with the other retrospective analyses.
A logical question is whether method of detection of recurrence has any effect on survival: no study has ever randomized patients to follow-up versus no follow-up, so level I evidence to address this question is lacking and only indirect observations can be made. Two studies have specifically addressed this question. Mooney and colleagues did not find any difference in overall survival between patients with asymptomatic (recurrences detected by clinicians on physical examination and/or laboratory/imaging investigations) versus symptomatic (patient-detected) recurrences. In their study, 72% of recurrences were detected by patients. Similarly, Francken and colleagues found no difference in survival in recurrences detected by patients versus recurrences detected by physicians. In their report, 73% of recurrences were detected by the patients, and most were symptomatic. In contrast, Poo-Hwu and colleagues reported a 5.8% improvement in overall survival for patients with asymptomatic recurrences discovered at routine follow-up, compared with symptomatic recurrences discovered by patients. However, biases in lead time (earlier detection that increases the time between diagnosis and death artificially) and length time (slow-progressing lesions with less aggressive potential are preferably detected), differences in the biologic nature of tumors discovered by patients versus health care providers, and differences in patients included in these analyses could account for these differences. In their prospective evaluation of melanoma recurrence, Garbe and colleagues stratified first recurrences as either early (asymptomatic) or late (symptomatic) and found a higher survival rate for patients with early versus late recurrences (76% vs 38% overall survival after 3 years). In this analysis, recurrences were not stratified based on who detected them (patient vs health care professional), and patients with all types of recurrences (first or subsequent) were included in their study and evaluation, once again limiting the ability to compare these results with other studies. Romano and colleagues specifically addressed site and timing for first relapse in stage III patients with melanoma. Their evaluation of 340 patients with a median follow-up of 77 months found overall recurrence-free survival rates for stage IIIA, IIIB, and IIIC of 63%, 32%, and 11%, respectively. Site of first relapse was local/in-transit in 28% of patients, regional nodal in 21% of patients, and systemic in 51% of patients. First relapses were detected by the patient in 47% of cases, clinically by the physician in 21% of patients, and by screening radiologic tests in 32% of patients. Multivariate analysis found that improved survival was associated with younger age, local/in-transit or nodal recurrence, asymptomatic recurrences, or resectable systemic recurrences. The investigators concluded that, based on the timing and site of relapse, routine physical examinations beyond 3 years for stage IIIA, 2 years for stage IIIB, and 1 year for stage IIIC and routine radiologic imaging beyond 3 years for stages IIIA and IIIB and 2 years for stage IIIC patients was of low yield and that published guidelines should strongly consider whether or not they should be included in recommendations for long-term follow-up.
Frequency of follow-up
Guidelines from most countries, melanoma cooperative groups, and consensus guidelines recommend follow-up visits with a physician who specializes in melanoma (surgical oncologist, dermatologist, or medical oncologist) with a complete history, physical examination (including skin and regional lymph node examination) every 3 to 12 months for the first 5 years after treatment of melanoma. Generally, follow-up is spaced out to every 6 to 12 months for 5 more years, with stage-specific frequency of visits. Table 1 summarizes the recommendations from 3 major multidisciplinary groups with expertise in melanoma (the National Comprehensive Cancer Network [NCCN, United States], the Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand, and the German Cancer Society) across 3 continents. The variability in follow-up frequency in these 3 recommendations is representative of other national and professional organizations.
Based on the consistent observations that most recurrences are detected by patients and that patient- versus clinician-detected recurrences do not affect survival, the concluding remarks from many studies suggest less frequent follow-up, although a specific time course outside major published guidelines is rarely suggested. For example, Francken and colleagues conclude that “the frequency of follow-up visits in an earlier SMU (Sydney Melanoma Unit) study and by other groups could be reduced for most patients.” They go on to remark, “However, individual patient-related factors such as physical and mental health, travel distance, and personal preferences will always need to be considered, and the extent of resources available to conduct follow-up programs may also be of relevance.” This statement summarizes the sentiment that many melanoma providers instinctually feel: that increased frequency follow-up does not affect survival and in the era of burgeoning practices and cost constraints, it is difficult to justify. A follow-up article recommends annual follow-up for stage I patients, 6-month follow-up for 2 years, followed by annual visits for stage IIA patients, and 4-month follow-up for 2 years, 6-month follow-up in year 3, and annual follow-up thereafter for stage IIB and IIC patients. As discussed in the preceding section, follow-up examinations and imaging tests were found to be of low yield beyond 3 years in patients with resected stage III melanoma.
In their prospective evaluation of follow-up, Garbe and colleagues adhered to the guidelines published by the German Society of Dermatology, which recommended follow-up examinations every 3 months in the first 5 years, with continued follow-up every 6 months until the 10th year. Based on the results of their study, the German Cancer Society adopted the guidelines presented in Table 1 , which slightly increased the follow-up intervals for early-stage melanoma.
One strategy to continue high-frequency follow-up, but relieve the burden of the melanoma specialist provider, is to transition patients to their primary care physician (general practitioner [GP]) for follow-up. A randomized trial of GP-led melanoma follow-up in the United Kingdom showed that such a strategy is safe, feasible, engenders greater patient satisfaction, permits closer adherence to guidelines, and does not result in adverse effects on health status or anxiety. There were 142 patients enrolled in this study over a 1-year period. Participating GPs were educated with a 4-hour training session and comprehensive literature for follow-up care, which focused on presentation of new and recurrent melanomas. Although the numbers are small, this study indicates that melanoma follow-up can be safely provided by any clinician, including physician extenders (such as nurse practitioners or physician assistants) educated in the timing and patterns of melanoma recurrence. This finding should not be surprising given the high likelihood that most recurrences are detected by patients.
Usefulness of laboratory and radiographic tests in the follow-up of the patient with melanoma
In the preceding sections, it seems that patient versus clinician detection of melanoma recurrences, along with the timing and frequency of follow-up visits, does not have an effect on survival. A logical question, then, is whether the addition of laboratory or imaging tests to clinic visits may be able to detect subclinical recurrences, which could then be treated with a combination of surgery, chemotherapy, and/or radiation therapy to improve outcomes. A central premise to this question is that earlier detection of recurrence improves survival. Although intuitively, oncologists often believe this hypothesis to be the case, there is no high-level evidence in melanoma to support it. Intensive follow-up with frequent laboratory and imaging studies, which have a significant false-positive rate, may be detrimental in terms of cost and patient-related anxiety. The next section reviews the evidence for inclusion of such studies in melanoma follow-up programs.
Laboratory Tests
As Table 1 indicates, the inclusion of laboratory studies during routine follow-up of the patient with melanoma varies across national guidelines. Historically, a complete laboratory panel (blood count, chemistry, liver enzymes, and lactate dehydrogenase [LDH]) was recommended in the follow-up of all patients with melanoma, but was not based on any evidence. The usefulness of this type of shotgun approach to the use of laboratory studies can be summarized as useless in the detection of recurrences, and detrimental in terms of cost and false-positive results, leading to subsequent futile tests. Even the usefulness of LDH, which has prognostic relevance in stage IV patients, and is incorporated into the staging classification for melanoma, does not have a role in the routine follow-up of stage I to III patients with melanoma.
The S-100B protein is currently used as a routine immunohistochemical marker in the diagnosis of melanoma and melanoma metastases, and serum levels of S-100B have been shown to be increased in patients with metastatic melanoma. Serum levels of S-100B have also been evaluated as a method of early detection of recurrent melanoma. In 141 patients followed for 15 months after complete resection of localized (stage I–II) melanoma, 7 patients experienced a recurrence, and in 6 of these 7 patients, rising serum S-100B levels preceded clinical or radiographic detection of metastases by 4 to 21 months. Similarly, Garbe and colleagues found that in patients with stage II to III resected melanoma, serum S-100B levels enabled earlier detection of distant metastases. In this study, 411 consecutive high-risk patients with melanoma were screened with S-100B serum levels. There were 41 recurrences and 13 (32%) of these patients had an increased S-100B serum level and in 8 (20%) of these, S-100B was the first sign of recurrence. This finding corresponds to a sensitivity and specificity of 32% and 96%, respectively, for the detection of metastases in this study group. A subsequent study by the same group found that S-100B had a sensitivity, specificity, and overall diagnostic accuracy for melanoma recurrence of 29%, 93%, and 84%, respectively. This finding was comparable with another serologic marker, melanoma-inhibitory activity (MIA) protein, which had a sensitivity, specificity, and overall diagnostic accuracy of 22%, 97%, and 86%, respectively. Both S-100B and MIA were superior to LDH and alkaline phosphatase, which had overall accuracy rates of 77% to 79%. In their prospective trial of melanoma follow-up, Garbe and colleagues obtained annual and biannual blood testing in the follow-up of stage I to II and stage III patients, respectively, which included blood counts, erythrocyte sedimentation rate, blood chemistries, liver enzymes, LDH, and S-100B (for the second half of the study, 1996–1998). They found that blood tests were rarely the first sign of metastases, and a first diagnosis was made in only 3 patients after further investigation after the detection of an increased LDH value. S-100B was increased in approximately half of patients with distant metastases and was the first sign of metastases in 25% of patients. However, S-100B was rarely increased in patients with locoregional recurrences and levels did not correlate with survival. Based on these results, the German Cancer Society guidelines recommend the measurement of serum S-100B levels in stage II and III patients with melanoma every 3 to 6 months during follow-up. These are the only major guidelines that recommend routine blood testing in follow-up of patients with melanoma. There is limited experience with S-100B outside Europe; thus, comparison studies are lacking.
The routine use of blood tests in the asymptomatic patient during follow-up for resected melanoma is not warranted. The low sensitivity, specificity, and accuracy for general laboratory profiles make them futile in the detection of subclinical recurrence. There may be a role for melanoma-specific markers (S-100B, MIA) in the follow-up of patients with melanoma, but this is not clearly defined. The lack of increase of these markers in locoregional recurrences, which may be treated for cure, further limits their usefulness.
Radiographic Tests
The role of routine radiographic testing as an adjunct to scheduled clinical visits to detect early recurrences follows the aforementioned unproven hypothesis that earlier detection of melanoma recurrences leads to improved survival. Although effective treatment of local, in-transit, and regional node metastases offers the possibility of cure, long-term survival after resection of systemic metastases is less frequent, but is possible. There are reports using chest radiograph (CXR), regional/nodal ultrasound (US), abdominal US, and computed tomography (CT) and magnetic resonance imaging (MRI) scans in the follow-up of patients with melanoma. Similar to studies mentioned earlier, the comparison of these studies is difficult given the heterogenicity of the patient cohorts, time frames of analysis, and definitions of recurrences. Further, with the exception of the single prospective study by Garbe and colleagues, all of these studies are retrospective in nature. However, the following studies represent the best data to date to guide our clinical practice. Each imaging modality is discussed in the following sections.
CXR and chest CT to detect pulmonary metastases
Screening for pulmonary metastases with CXR and CT scans in patients with melanoma is based on the observation that the lungs are the most common visceral site of metastases. Of patients with pulmonary involvement, 12% to 25% are eligible for surgical resection, with 80% to 90% of these patients undergoing complete (R0) resection. The 5-year survival in this highly selected group can be as high as 30%. Thus, the goal of radiographic screening tests to detect pulmonary metastases is to detect asymptomatic lesions at an earlier stage that are amenable to an R0 resection. Ideally, a randomized trial of routine pulmonary imaging versus no imaging could answer the question of whether these tests improve survival; however, these studies do not exist. CXR is the most studied test to date. In terms of detection of pulmonary metastases, there are mixed findings in the published literature concluding that CXR is able or unable to detect asymptomatic pulmonary metastases from melanoma. In the studies that found that CXR was able to detect pulmonary recurrences, the rate was 0.5% to 6.2% of patients. The wide variability reported in these studies can be explained by the wide range of patients included and the differing length of follow-up. For example Kittler and colleagues and Bassères and colleagues specifically analyzed patients with thin melanomas, whereas studies by Kersey and colleagues, Goerz and colleagues, and Mooney and colleagues each included more high-risk stage II and III patients in their analyses. No study has shown that the early detection of pulmonary metastases by routine imaging is associated with improved survival. Taso and colleagues examined 994 patients at the Massachusetts General Hospital and found that 75 (8%) patients had pulmonary metastases detected by CXR (1937 total CXRs). In addition, there were 63 (6% of patients) with false-positive results. In 41 patients (4%), CXR was the initial evidence of metastases. Survival after identification of pulmonary metastases did not differ between patients with asymptomatically detected pulmonary metastases versus those with symptomatic or pulmonary metastases found in the setting of other metastatic sites. Meyers and colleagues found that in 118 patients with stage II or III melanoma followed with routine clinical examinations and imaging tests over at least 2 years, only 2 of 43 recurrences (5%) were detected by CXR. At the SMU, Morton and colleagues enrolled 108 patients with stage III patients in a prospective monitoring schedule of CXR every 6 months for 5 years. Patients were followed for a median of 52.5 months, and 23 (21%) developed pulmonary metastases, of which 11 (48% of recurrences) were detected by CXR. Three of the 23 patients with pulmonary recurrences detected by CXR (13% of total) went on to resection. Further, abnormal CXRs were found in 19 additional patients, but not because of recurrence (false-positive result). Median survival did not differ between recurrences detected by CXR and those not detected by CXR. The investigators concluded that because routine CXRs detected only half of pulmonary recurrences, infrequently identified patients for potentially curative surgery, had a high rate of false positivity, and did not affect survival, their use was not justified. In Garbe and colleagues prospective study of melanoma follow-up, CXR detected metastases in 2 patients (11.1%) with stage I melanoma, 11 patients (22.4%) with stage II melanoma, and 13 patients (9.5%) with stage III melanoma. Overall, 0.6% of all CXRs were responsible for the detection of metastases, and as many as 75% of recurrences detected by CXR were first discovered at an advanced phase. These investigators concluded that the routine CXR was of little benefit for stage I and II patients and should be restricted to follow-up of patients with more advanced stages of disease.
Mooney and colleagues performed a cost-effectiveness analysis using data from the Roswell Park Cancer Institute and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. They found that the cost of CXR screening per nonquality-adjusted life year and quality-adjusted life year were $150,000 and $165,000, respectively, in 1996 US dollars. This amount accounted for 80% of program costs. Based on these findings, the investigators suggested reducing the frequency of screening CXR.
In an effort to improve the usefulness of CXR in detecting pulmonary recurrences of melanoma, 3 studies have evaluated the use of chest CT scans. Only one identified metastases, but in only 3 of 364 patients with stage I to III melanoma, 2 of whom had distant metastases elsewhere. However, these studies are limited; the study by Bassères and colleagues evaluated only patients with stage I melanoma, whereas the study by Mijnhout and colleagues comprised only 67 patients with resected stage I to III melanoma, 51 of whom (76%) underwent screening CT scans. Given these results, along with the inability of screening CT scans to readily detect primary lung cancers in heavy smokers without an unacceptable rate of false-positive results, this modality does not seem appropriate for general use in the follow-up of the patient with melanoma.
The overall sensitivity, specificity, false-positive, and false-negative rates of studies examining screening CXR in the follow-up of patients with melanoma is presented in Table 2 . The inability of this testing modality to detect recurrences and affect survival has led to the omission of routine CXR in the NCCN and Australia/New Zealand guidelines and the limited use of CXR only in stage II and III patients in the German guidelines (see Table 1 ).