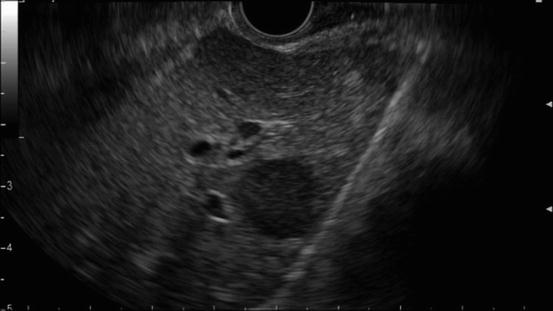
Fig. 2.1
A typical example of portal vein invasion on EUS. The finding “loss of the vessel-parenchymal interface” is observable
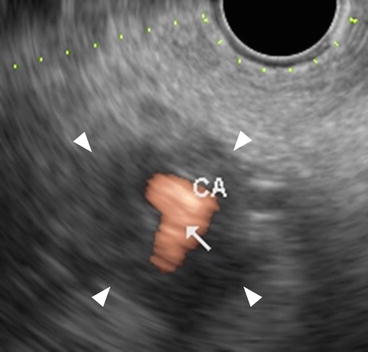
Fig. 2.2
A typical example of celiac artery invasion on color Doppler EUS. The finding “abnormal vessel contour” is observable
When the accuracy of EUS for diagnosis of vascular invasion by pancreatic cancer is compared with that of CT, the values should be separately compared for each vessel (PV, SMA, CA) because observation with EUS depends on the location of the vessels [4, 6, 8, 11, 12]. Tumor size may also affect the accuracy of EUS staging. Attenuation of the ultrasound beam in large tumors lowers the accuracy. For this reason, tumors below 3 cm in size are more accurately staged with EUS [13]. Unlike radial EUS, linear EUS can show arterial vessels longitudinally with a linear image, and both the superior mesenteric and celiac arteries are more easily followed from the stomach with linear EUS. However, a recent meta-analysis of 29 studies on vascular invasion revealed no significant differences in the accuracy of radial and linear examinations [11].
In a recent meta-analysis assessing the performance characteristics of EUS in the staging of pancreatic cancer, which included 49 studies, the accuracy of EUS in the detection of vascular invasion ranged from 62 to 100%, with a pooled sensitivity of 85% and specificity of 91% [9]. EUS appeared to be more sensitive for detecting vascular invasion than CT although both CT and EUS revealed comparable specificities [9]. EUS has also been demonstrated to offer better results than angiography [14, 15]. EUS had a higher sensitivity for the detection of vascular involvement than selective venous angiography (86% vs 21%, respectively; p = 0.0018). The specificity and accuracy of EUS for detecting vascular involvement were 71% and 81%, respectively, while for selective venous angiography they were 71 and 38% [15].
In examinations according to vessel type, the sensitivity of EUS for tumor invasion of the portal vein (PV) has been reported as superior to that of CT [8, 12, 16, 17] and angiography [4, 8, 12, 16]. By contrast, EUS has shown low sensitivity in the SMV, SMA, and CA [3, 12, 17, 18]. Reported values for the sensitivity of EUS for tumor invasion of the PV range from 60 to 100%, with most studies demonstrating sensitivities over 80% [4, 6, 8, 12, 16, 19]. Yasuda et al. regarded a “rough-edged vessel with compression” as a marker of tumor invasion, and, using this criterion to evaluate tumor invasion of the PV, they found a sensitivity, a specificity, and an accuracy of 79%, 87%, and 81%, respectively [6]. Rösch et al. used “abnormal contour, loss of hyperechoic interface, and close contact” as a definition of tumor invasion, and using these criteria they found a sensitivity and specificity for the evaluation of tumor invasion of the PV of 43% and 91%, respectively.
Although the National Comprehensive Cancer Network guidelines for pancreatic cancer handle the invasion of the PV and arteries [20] separately, there are few reports that specifically examine the invasion of the CA; there may be several reasons for this. Firstly, it is difficult to obtain confirmation from surgical findings because in most cases where imaging modalities showed the invasion of the CA, the patient did not undergo surgical resection. Secondly, it is difficult to obtain histologic correlations with intraoperative findings in regard to vascular invasion. One report found the sensitivity of EUS for tumor invasion of the CA to be 57% although the sensitivity for tumor invasion of the SMA was only 17% [12]. A limitation of the radial scanning method employed with incomplete visualization of the SMA, and the sensitivity of EUS was found to be lower than that of CT. EUS evaluation of the SMA may be technically difficult due to either the inability to visualize the entire course of the vessel, or obscuration of the vessel by a large tumor in the uncinate or inferior portion of the pancreatic head [8].
Contrast-enhanced harmonic EUS (CH-EUS) has been developed for the diagnosis of pancreatic cancer; however, there are few reports on its use for the evaluation of vascular invasion by pancreatobiliary diseases. Imazu et al. evaluated CH-EUS for T-staging in 26 patients with pancreatobiliary carcinomas and reported that the overall accuracy for T-staging of CH-EUS (92%) was significantly higher than that of conventional harmonic EUS (69%; p < 0.05) [21]. Further studies are warranted to confirm the utility of CH-EUS for T-staging of pancreatic cancer.
2.3 EUS Diagnosis of Node Metastasis in Pancreatic Cancer
As lymph node stage relates not only to the choice of treatment, but also to the prognosis, it is essential that the techniques used for N-staging are reliable [22, 23]. Pancreatic cancer patients with para-aortic lymph node (PALN) metastases have a poor prognosis [24]. A previous article reported that, in a multivariate analysis, the presence of PALN metastases was an independent factor with a significant association with mortality, and that 84% of those patients with positive PALN metastases died within 1 year [25]. If PALN metastases are detected, alternative treatment strategies should be considered. However, the diagnosis of malignant intra-abdominal lymph nodes is often challenging for endoscopists and radiologists [26]. A previous article evaluated the efficacy of ultrasonography (US), computed tomography (CT), endoscopic ultrasonography (EUS), and magnetic resonance imaging (MRI) in the assessment of lymph node metastases in pancreatic carcinoma, and EUS had an independent predictive value for tumor metastases in regional lymph nodes [27].
In EUS examinations, lymph nodes have been evaluated in terms of their size (i.e., the short and long axis lengths), shape (round or oval), edge characteristics (sharp or fuzzy), and echogenicity (hypo or hyper). A recent study reported that a short axis of 13 mm or longer and a long axis of 20 mm or longer had the best sensitivity and specificity for predicting malignancy [28]. Additionally, a round shape, a sharp edge, and hypoechogenicity were also found to be reliable parameters for predicting malignancy. Most studies have found no difference between CT and EUS in the prediction of resectability in relation to node involvement [6, 14, 29–31]. Only one study found EUS to be superior to CT for N-staging (EUS 93.1% vs CT 87.5%). Sawhney et al. [32] reported that the absence of a central intra-nodal vessel on color Doppler EUS is a strong and independent predictor of a metastatic lymph node (Fig. 2.3) although another article reported that the absence of such a vessel (Fig. 2.4) on color Doppler EUS did not predict malignancy better than the standard EUS variables [28].
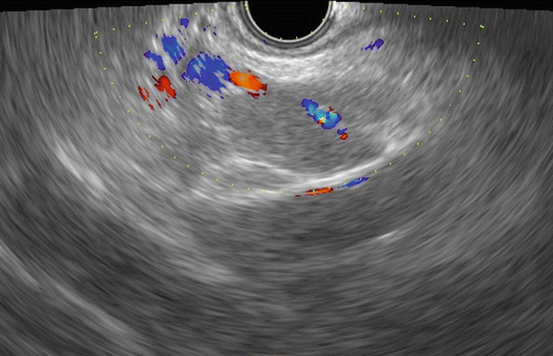
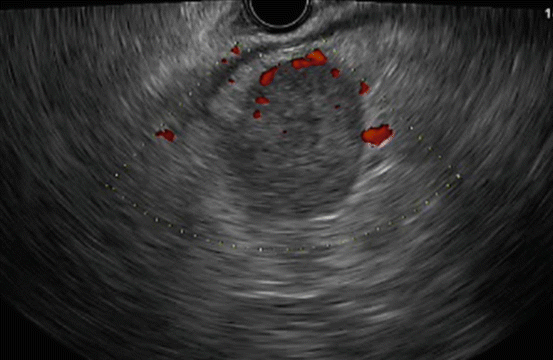

Fig. 2.3
A typical example of “central intra-nodal vessel” presence on color Doppler EUS

Fig. 2.4
A typical example of “central intra-nodal vessel” absence on color Doppler EUS
Several studies have reported that although EUS (which has good spatial resolution) is useful for the differential diagnosis of malignant and benign lymph nodes, its diagnostic accuracy remains unsatisfactory [32–34]. By contrast, a cyto-pathological diagnosis via endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is highly accurate. A recent study compared EUS-FNA and [18F]-Fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) for the diagnosis of PALN metastases [35], and found EUS-FNA to be superior to PET/CT for preoperative PALN staging in patients with pancreatobiliary cancers. Because of the clinical benefits of EUS-FNA for reducing unnecessary surgery, it should be considered a part of the standard preoperative examination for patients with pancreatobiliary cancers.
As EUS-FNA is highly accurate for the identification of malignant lymph nodes, most cases in which EUS-FNA reveals the presence of atypical cells in the lymph nodes do show malignancy of the lymph nodes [36]. However, false-positive and false-negative EUS-FNA results remain possible. Jason et al. [37] reported EUS-FNA false-positive and false-negative rates for intra-abdominal lymph node diagnosis as 0.7% and 5.8%, respectively. Additionally, EUS-FNA has another limitation, in that it cannot be performed in all cases, because of intervening vessels and/or the difficult location of the lymph node; this could, for example, lead to an excessively large scope angle or distance from the probe.
Thus, an accurate alternative evaluation method is needed for cases in which a lymph node cannot be accessed by EUS-FNA, or where EUS-FNA does not obtain adequate material for analysis. One such alternative method is EUS elastography. EUS elastography has been presented as a novel technique to assess tissue elasticity and has been used to differentiate between malignant and benign lymph nodes. Several different variables have been used as a measure of tissue elasticity in EUS elastography, including color patterns [38–43], strain ratio [44, 45], hue histogram analysis [46, 47], and computer analysis with artificial neural networks [48, 49]. Xu et al. [50] performed a meta-analysis that included seven articles and a large number of lymph nodes (368 patients with 431 lymph nodes). The sensitivity and specificity of EUS elastography were 88% and 85%, respectively, for the differential diagnosis of benign and malignant lymph nodes. The area under the summary receiver operating characteristic curve was 0.9456. However, the sensitivity and specificity of this method have varied greatly between studies [50].
Another alternative method is contrast-enhanced color Doppler EUS with a US contrast agent. Kanamori et al. [51] reported that, with the first generation of US contrast agent (Levovist; Nihon Schering Co., Ltd., Tokyo, Japan), defective enhancement on contrast-enhanced color Doppler EUS predicted lymph node malignancy significantly more accurate than standard EUS variables although the method suffered from Doppler-related artifacts. Recently, the combination of second-generation US contrast agents, including Sonazoid, SonoVue, and Definity, and low mechanical index imaging techniques, has led to CH-EUS being used for perfusion imaging, which facilitates the depiction of tumor vascularity [52–55]. The second-generation of US contrast agents resonate with a low acoustic power, and thus allow CH-EUS to be performed. A previous report demonstrated this method to have an excellent ability to differentiate malignant from benign lesions, without Doppler-related artifacts, even when lesions were small [56]. In a recent study [28], heterogeneous enhancement was observed in 39 of 47 (83%) malignant lymph nodes, and CH-EUS had a significantly higher diagnostic accuracy for malignant lymph nodes than most of the standard EUS variables (Figs. 2.5 and 2.6). The study also showed that CH-EUS was comparable to EUS-FNA for N-staging (88% vs 90%, p = 0.50). Additionally, in all the cases where EUS-FNA failed due to inadequate sampling or inaccessibility of the lymph nodes, CH-EUS resulted in correct N-staging. Thus, CH-EUS may be a useful modality for differentiating malignant from benign lymph nodes in patients with pancreatobiliary carcinomas, and may complement standard EUS, color Doppler EUS, and EUS-FNA, all of which have some limitations. It may also be helpful for determining which lymph nodes should be subjected to EUS-FNA. In view of its high accuracy, CH-EUS may help to avoid unnecessary surgery in the future. Hence, CH-EUS will play an important role in determining the optimal treatment for pancreatobiliary carcinomas.
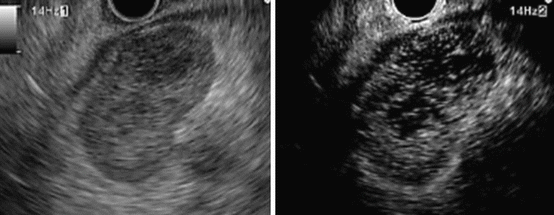
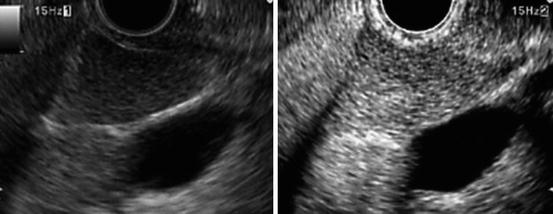

Fig. 2.5
A typical example of a lesion with heterogeneous enhancement (metastatic lymph node; the long axis is 25 mm and short axis 15 mm). Standard EUS (left) shows a round shape and sharp edge. CH-EUS (right) shows that this area exhibits heterogeneous enhancement

Fig. 2.6
A typical example of a lesion with homogeneous enhancement (benign lymph node; the long axis is 28 mm and short axis 18 mm). Standard EUS (left) shows an oval shape and sharp edge. CH-EUS (right) shows that this area exhibits homogeneous enhancement
2.4 EUS Diagnosis of Liver Metastases in Pancreatic Cancer
Most pancreatic cancers will develop liver metastases during the course of the disease [57]. Early detection of liver metastases in patients with known pancreatic cancer is important for therapeutic decision-making and is crucial to the prognosis for survival. In those patients who develop a recurrence following an apparently curative pancreatic resection, the high frequency of liver recurrence rates indicates that these metastases were present at the time of surgery, but remained “occult,” i.e., undetected by preoperative imaging examinations. It is well recognized that patient outcome is highly dependent upon the ability to define the true extent of the metastatic disease; therefore, it is crucial to have a preoperative imaging modality with a high sensitivity for the detection of liver metastases. Traditionally, transabdominal US, and contrast-enhanced CT and/or MRI, have been used for staging in patients with pancreatic cancer, particularly for surveillance of liver metastases [58–60]. Unfortunately, these modalities are limited in their ability to detect liver lesions of less than 10 mm in diameter [61].
EUS was not traditionally thought to be clinically applicable to liver imaging until the first report appeared in 1999 [62]. Under EUS, the liver could be detected from the proximal stomach and/or distal esophagus to view the left lobe, and from the gastric antrum and/or duodenal bulb to view the portal hilum and proximal right lobe, including the major portion of the intrahepatic biliary tract. According to Bhatia et al., most liver segments can be visualized with EUS. The intrahepatic vascular landmarks include the major hepatic veins, PV radicals, and hepatic arterial branches, while the inferior vena cava, venosum, and teres ligaments form other important intrahepatic landmarks. The liver hilum and gallbladder serve as useful surface landmarks [63]. The caudate lobe lies in close proximity to the stomach and duodenum; therefore, EUS is superior to transabdominal US for imaging these regions. Conversely, EUS is limited in its ability to access the portion of the right lobe adjacent to the dome of the diaphragm, along with its lateral and inferior portions [64].
Nguyen et al. prospectively evaluated EUS images of the liver in 574 consecutive patients with a history or suspicion of gastrointestinal or pulmonary malignant tumors. Fifteen liver lesions (in 14 patients) were identified (5 were in the right lobe, 9 were in the left lobe) and underwent EUS-FNA; 14 of these were confirmed as malignant. Moreover, CT depicted liver lesions in only 3 of these 14 patients before EUS, with 12 of the 15 lesions being less than 20 mm [62]. Likewise, a retrospective study conducted by Prasad et al. revealed that EUS detected metastatic lesions missed by conventional cross-sectional imaging studies in 5 of 222 cases (2.3%), with EUS having sufficient resolution to detect lesions as small as 5 mm in diameter, although the technique was more operator dependent than other imaging modalities [65]. Awad et al. evaluated 14 consecutive patients with a history of a known liver mass. The patients underwent both contrast-enhanced CT and EUS. EUS not only allowed identification of the lesions in all 14 patients, but also led to the identification of four new lesions smaller than 5 mm, which had not been visualized by the preceding CT scan [61]. These studies suggest that EUS is a useful modality for detection of liver metastases, particularly small lesions.
Liver biopsy has traditionally been performed percutaneously under transabdominal US or CT guidance, and a transjugular fluoroscopy-guided approach is applied when a percutaneous approach is contraindicated because of coagulopathy or ascites [66]. Recently, liver biopsy under EUS guidance has emerged as an alternative to the percutaneous or transjugular approach, and its high diagnostic accuracy and safety have been described in many articles [61, 62, 65, 67–76]. EUS has an advantage over the other noninvasive imaging methods (CT or MRI), in that samples for pathological diagnosis can be obtained by subsequent EUS-FNA. In 2002, ten Berge et al. conducted a large retrospective international multicenter survey to evaluate the indications, complications, and findings of EUS-FNA of the liver [67]. The study revealed that the complication rate for EUS-FNA of the liver was only 4%, with a major complication rate of 1% in 167 cases. They also showed that EUS-FNA helped to diagnose malignancy in cases that could not be accessed by transabdominal US-guided FNA. The authors therefore concluded that EUS-FNA of the liver was a safe procedure, and that EUS-FNA should be considered when a hepatic lesion is difficult to access with transabdominal US or CT-guided FNA, or when these modalities are unable to result in a diagnosis [67]. Singh et al. prospectively compared the accuracy of EUS/EUS-FNA and CT scan for the detection of liver metastases, and revealed that there was a trend in favor of EUS/EUS-FNA, with a superior diagnostic accuracy in comparison with CT [71] (Fig. 2.7). A large multicenter prospective clinical trial that included 110 patients showed that the rate of definitive pathological diagnoses was 98%, and the overall complication rate was only 1%, which led the authors to conclude that EUS-FNA of the liver was a safe technique which provides a high diagnostic accuracy [75].