CH etiology
Associated mutations
TSH
T3 and T4
A. Primary CH
Thyroid dysgenesis
PAX8
high
low
TTF2
NKX2.1
NKX2.5
Thyroid dyshormonogenesis
NIS/SLC5A5
high
low
TPO
DUOX2, DUOXA2
Tg
DEHAL1/SECISBP2
SLC26A4
Resistance to TSH
TSH-R
high
low
Gs alpha
B. Central (secondary) hypothyroidism
Congenital hypopituitarism
HESX1
low
low
LHX3
LHX4
PIT1
PROP1
Isolated TSH deficiency
TSH b
low
low
TRH deficiency
low
low
TRH resistance
TRH-R
low
low
C. Peripheral hypothyroidism
Thyroid hormone resistance
THRb
normal/high
mildly high
Abnormalities of thyroid hormone transport/metabolism
MCT8
normal
high T3, low T4
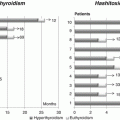
Transient CH may be caused by maternal or neonatal factors. Maternal factors include exposure to iodine deficiency or excess (amiodarone or iodine antiseptic compounds), transplacental passage of thyrotropin receptor blocking antibodies, and use of antithyroid medications by mothers with hyperthyroidism. Neonatal factors include neonatal iodine deficiency or excess [2], congenital liver hemangiomas (with increased deiodinase type 3 activity), and mutations in the genes DUOX2 and DUOXA2.
Permanent primary CH in iodine-sufficient areas is caused in 85 % of cases by thyroid dysgenesis, resulting from an aberration of the embryological development of the thyroid gland.
Thyroid dysgenesis encompasses three major forms, thyroid ectopy, athyreosis, and hypoplasia, accounting for 30–45 %, 35–45 %, and 5 % of cases, respectively [3–5]. Thyroid ectopy refers to an ectopic location of the thyroid gland, inferior or superior to the hyoid bone or above the thyroid cartilage. A thyroid remnant is usually found along the normal pathway of the thyroglossal duct [6, 7]. Athyreosis refers to the complete absence of thyroid tissue and thyroid hypoplasia to an aberrant development resulting in reduced thyroid volume. Thyroid dysgenesis is generally sporadic in occurrence. However, a genetic component is evident in about 2 % of cases [8]. Genes implicated as a cause of thyroid dysgenesis include thyroid transcription factor 2 (TTF–2) [9], NKX2.1 (also known as TTF–1) [10, 11], NXK2.5 [12], and paired box gene eight (PAX8) [13, 14]. These genes encode for transcription factors expressed both during thyroid embryogenesis and in the normal functioning gland. They are also present in other tissues and are associated with syndromic CH. Thus, patients with homozygous missense mutations in TTF–2 present with spiky hair, cleft palate, cohanal atresia, and thyroid dysgenesis (Bamforth-Lazarus syndrome) [9]. Mutations in NKX2.1 cause CH associated with neonatal respiratory distress, ataxia, and chorea [10, 11]. In patients with NKX2.5 mutations, CH is associated with cardiac malformations [12]. In the presence of PAX8 mutations, genitourinary malformations may be detected along with CH [15, 16].
About 15 % of cases of permanent primary CH are caused by dyshormonogenesis, encompassing various defects in thyroid hormone production. The defects are usually inherited in an autosomal recessive manner and include mutations in genes encoding the sodium-iodide symporter (NIS) (SLC5A5 gene), thyroperoxidase (TPO), hydrogen peroxide generation factors, i.e., thyroid oxidase, and dual oxidase maturation factors (DUOX2 and DUOXA2 genes), thyroglobulin (Tg), iodothyronine deiodinases (DEHAL1 or SECISBP2 genes), and the transmembrane protein pendrin, which acts as a chloride-iodide transporter both in the thyroid and in the inner ear (Pendred’s syndrome, due to SLC26A4 gene mutations, is characterized by hypothyroidism, goiter and deafness) [17].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree