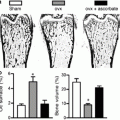
Fig. 3.1
Groups of male mice ranging in age from 4 to 6 months were studied. Control mice (CON, n = 11) were hormonally intact while those in the aromatase knockout (ARKO, n = 9) group were deficient in E2. The muscle masses of two representative muscles, gastrocnemius and tibialis anterior, are presented. The mass deficiency for the gastrocnemius (17 %) and tibialis anterior (20 %) is significant (p < 0.01). Force per muscle mass was comparable between groups
When the WHI study was terminated prematurely, millions of women were taken off of HRT and E2 alone due to the potential negative side effects of sex hormone therapy. Since that time it has been realized that the WHI data presented a very incomplete picture of hormonal effects on peri- and postmenopausal women but clinical practitioners are still reluctant to prescribe E2. There are other estrogenic compounds that potentially can stimulate skeletal muscle, possibly through ER, compounds such as plantlike estrogens (phytoestrogens). Soy-based products have been evaluated to a modest extent and it appears that soy isoflavones are weakly estrogenic (anabolic), particularly when combined with exercise. Future research should yield additional insights into other phytoestrogens, synthetic steroids, and specific selective estrogen receptor modulators, compounds that may have the desirable effects on skeletal muscle (and bone) without any deleterious side effects.
Estrogen Effects on Muscle Strength
The inevitable outcome for women who have less muscle mass is less muscle strength. Unfortunately, the typical association of mass and strength is lost in women who are E2 deficient. There is ample evidence, from both the human and animal literature, indicating that E2 impacts muscle quality, specifically force/unit area, such as muscle mass or cross-sectional area [12, 16–19]. Thus, with E2 deficiency, a greater amount of force than skeletal muscle is lost, which may explain the decline in functional capacity in many postmenopausal women. A recent meta-analysis of the human and animal literature indicated that muscle force/unit of muscle (specific force) is approximately 10 % less in the ovariectomized (OVX) rodent, although results from a variety of studies range from no change to almost 20 % less specific force [18]. The cause(s) associated with the loss in force/unit area is unclear. Moran et al. determined, using electron paramagnetic resonance spectroscopy, that the fraction of strong-binding myosin was ~15 % less in EDL single fibers from OVX mice [16]. The loss of strong binding was consistent with the decline in EDL specific force (19 %). These findings suggested to the investigators that the loss of ovarian hormones causes a loss in the total number of actin and myosin molecules, a reduction in the fraction of myosin that is strongly bound to actin during the contraction cycle or, possibly, that the force generated per molecule of myosin is reduced [16]. These results could potentially explain the lower specific force but findings have never been replicated.
To put the loss of specific force in context, van Geel et al. reported in 329 healthy postmenopausal women between the ages of 55 and 85 years (cross-sectional study) that muscle mass loss was 5 % when those in the 55–65-year-old group were compared to the 75–85-year-old women [20]. Within the same comparison groups the decline in maximal knee extension strength (32 %) and grip strength (23 %) far exceeded the loss in lean mass, suggesting a dramatic decrease in muscle efficiency with age, possibly compositional change in lean mass. The declines in strength (and lean mass) were associated with bioavailable serum E2 levels. Although all of the women were aging, it was not possible to separate age-related muscle decline from the changes associated with loss of ovarian hormones. Nonetheless, findings strongly support the importance of active E2 (and testosterone).
Muscle force loss was also measured in the Finnish twin study. To reiterate, 13 postmenopausal monozygotic twin pairs were identified, one of whom had taken HRT through the menopause (average duration of use was 6.9 years) while the other twin had not [12]. The twin taking HRT had more muscle mass and significantly more mobility than the twin not taking HRT. Lower body muscle power measured as vertical jump height was 16 % higher in the HRT users. Average walking speed was 7 % greater in women taking hormones. This study is important for two reasons: first, the use of monozygotic twin pairs eliminates biologic subject variability and second, the investigators demonstrated the functional consequences of lost ovarian hormones. Older women are far more likely than men to require nursing home placement due to loss of functional mobility. Findings from the twin study suggest that the loss of E2 plays a role in the decline in physical function.
E2 and Exercise Effects
Because the loss of ovarian hormones results in a decline in muscle mass and strength, it begs the questions of whether strengthening exercise can offset or mitigate these losses. Surprisingly, little is known about the effects of exercise alone in the perimenopausal women or if there is an interaction of E2 and exercise. Several studies have reported that the postmenopausal woman does not gain strength to the same magnitude as men or premenopausal women, suggesting a role for E2 to augment muscle force increases with exercise. Our lab strength-trained older men and women (average age 82.3 years) at 70–80 % of 1-RM, 3x/week, for 3 months and found the overall increase in strength for the men (67 %) to be significantly higher than the strength increase for older women (45 %) even though both groups worked at the same relative intensity (Fig. 3.2). However, Petrella et al. studied young (20–29 years) and older men and women (60–75 years, mean age 63.7 years) before and after 4 months of rigorous strength training for the knee extensors [21]. Biopsies were taken from the vastus lateralis muscle before and after training and myofiber cross-sectional area determined. Hypertrophy occurred in all groups (young and older men, young and older women) but the greatest increase occurred in the young men (~32 %). Younger women had an ~32 % increase in strength whereas older women showed a 25 % improvement. Curiously, the number of myonuclei per muscle fiber increased only in the young men. The number of NCAM-positive cells (indicative of satellite cell incorporation) was higher only in the young men. These findings suggest that there is little difference in training adaptation for young vs. older women. Whether these two studies are comparable given a nearly 20-year difference in age for older subjects remains to be seen. One important point to emerge from both of these studies is that older postmenopausal women are adaptable to strength-training and capable of making gains in muscle mass and force, whether the magnitude of change is the same or lower than that of younger women. Gains in muscle mass and strength are important for the maintenance of independence in old age.
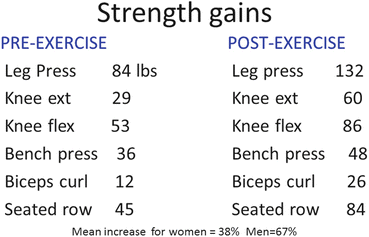

Fig. 3.2
Strength gains following 3 months of traditional training at 60–80 % of 1-repetition maximum. On average, participants trained rigorously 2 days/week and stretched or worked with therabands during a third session. The training stimulus was the same for both men and women
Several additional studies provide results that are challenging to interpret and it is still unclear whether E2 augments gains in muscle mass and strength with training in the woman who is hormone deficient. Technically, exercise and E2 effects should be additive if there is an independent hormone augmentation of muscle during exercise. In one study of postmenopausal women Sipila et al. (2001) randomized their 50–57-year-old subjects into exercise, exercise-plus-HRT, HRT-only, and untrained control (placebo HRT) groups. Women in the exercise groups strength-trained 2–3 days/week for 12 months [22]. Explosive power significantly increased in the HRT–exercise and HRT groups. Knee extension torque and muscle fiber cross-sectional area also increased in the HRT–exercise and HRT-alone groups. While the differences between these two groups were not significant, the magnitude of increase in muscle torque and fiber area in the combined HRT–exercise group was greater than that observed in the HRT-only subjects suggesting an additive beneficial effect of hormone replacement. Subject variability was substantial which likely masked potential differences given subject numbers in each group. Subsequent analyses of the same women [23] revealed that quadriceps and posterior compartment (e.g., hamstrings) muscle loss was attenuated in women taking HRT compared to controls. In a separate study Brown et al. (1997) strength-trained 42 women, half of whom were on HRT. Women were between the ages of 60 and 72 and all of them exercised 3 days/week. The first three months were spent in stretching, balancing, and light calisthenics in preparation for the subsequent 6 months of strength training at ~75 % of 1-RM. At the end of 9 months strength was significantly increased in leg press, hip extension, and isokinetic knee extension/flexion. However, the gains made by the HRT-plus-exercise group were no greater than the gains made by women who were not on HRT, which raises the question of whether women were too far beyond the menopause to benefit from hormonal intervention [24]. Regardless, studies of strength training in postmenopausal women indicate that they are capable of making significant gains in muscle strength. Whether the increases in muscle mass and strength were augmented by HRT seems unclear but few studies have addressed this issue and more are needed.
One rodent study of exercise and E2 effects on skeletal muscle warrants mention. Grieseing et al. (2011) determined if E2 effects on skeletal muscle were independent of physical activity. To that end they randomized mice to groups that had increased physical activity (wheel running), diminished activity (HLU for 2 weeks), and no muscle activity (nerve transection). In all instances, E2 effects on muscle force were independent of activity level [25]. Soleus muscles in OVX mice from the HLU and nerve transected groups had 31 % less muscle force/muscle protein content than mice subjected to the same conditions but supplemented with E2. Findings strongly support the concept that E2 significantly influences muscle power independent of physical activity levels.
E2 Effects on Muscle May Be Fast Acting or Exerted Through ER
The apparent anabolic effects of E2 appear to be mediated through the ER. In rat myotubes [26] Wiik et al. studied first whether ER were present and secondly whether ER expression increased with E2 and electrical stimulation (exercise). Investigators first demonstrated that both ERα and ERβ are present in myotubes. Next E2 was used to determine if mRNA levels of ERα and ERβ would increase in response to hormone stimulation. In their cells only ERβ increased in response to E2 while ERα remained unchanged. In a separate set of studies Galluzzo et al. studied the effects of E2 on ERα and ERβ using rat myoblast L6 cells [27]. In their cells, Akt activation was the consequence of ERα stimulation, not ERβ. Akt stimulation has been linked to muscle hypertrophy and muscle development. Both receptors ERα and ERβ were involved in E2-mediated activation of p38. The p38/MAPK pathway has been identified as critical for muscle cell differentiation and the fusion of myoblasts into myotubes, a key step in muscle regeneration. Whether these studies are complementary is unclear. A third ER has been postulated which may modulate ER activity. This putative third receptor is called Gper and is expressed in both the soleus and EDL muscles. Gper is responsive to E2 and appears to be associated with antioxidant gene expression [28]. More study is needed to better understand the potential role of Gper in muscle function.
To further elaborate the role of ER, our laboratory studied female knockout (KO) mice without ERα or ERβ. Muscle mass and contractile tensions were determined for four separate muscles with different anatomic and fiber type profiles. Muscle mass was essentially unaffected in ERα and ERβ KO females. Peak tetanic tension/anatomical cross-sectional area was significantly reduced in the ERα KO gastrocnemius and tibialis anterior but not in the plantaris or soleus muscles. The absence of ERβ had no impact on muscle force. Total myosin content was unaffected by KO status [19].
These studies suggest that the ERs are important determinants of pathway activation in response to E2. Receptor function has been studied extensively but not in skeletal muscle, so the role of ER in muscle has not been precisely determined. What has become apparent recently in other cell types is that ERβ can act as a negative or a positive regulator of ER activity and that both receptors can be involved simultaneously as α/β heterodimer which may explain some of the results that seem contradictory [29–31]. More study of nuclear ER involvement is needed.
17-β estradiol can also stimulate non-transcriptional responses by non-genomic signaling. Activation of the PI3K/Akt and MAPK pathways, for example, can occur with or without the classic stimulation of the estrogen response element in the nucleus. The non-genomic effects of E2 are hypothesized to activate receptors outside the nucleus such as those on the cell membrane, in the cytoplasm, and in the mitochondria. Non-genomic signaling has been found to increase the expression of a variety of signaling molecules including ERK1/2, JNK1/2, p38, CREB, and c-fos. Cellular processes associated with non-genomic signaling include the regulation of apoptosis, mitochondrial function, and blood flow to muscle [26, 32].
E2 Effects on Gene Expression
Several studies have addressed the question of what happens to muscle when E2 is absent or when an E2-deficient organism is given E2 treatment. Overall, results indicate that E2 has direct effects on gene expression in a variety of ways but most effects still need to be elaborated in more detail.
Cell culture experiments provide strong evidence of E2 effects on gene expression. Wiik et al. [26] cultured rat myoblasts (curiously, from one male rat) and once cells reached confluency, they were transfected with estrogen response element (ERE)—luciferase. Cells were subsequently differentiated to myotubes and either stimulated to contract (exercise) or exposed to ICI 182,780, an ER antagonist, or the MAPK inhibitor PD-98059. Electrical stimulation activated the ERE-LUC reporter construct. To determine if activation was ER dependent, ICI 182,780 was used. Activation of the ERE was unaffected by ICI 182,780 but activation was abolished when the MAPK inhibitor PD 98059 was added to the cell culture. E2 also stimulated ERE-LUC activity but activation involved the ER. Thus, results indicate that exercise and E2 stimulate ERE activation but one involves the ER and the other does not.
Kahlert and colleagues [31] cultured L6 and C2C12 myoblasts with 17-β estradiol or estrone and determined if there is evidence of E2 effects on skeletal muscle growth. Gene transactivation, as evidenced by activation of ERE-LUC, did occur in response to 17-β estradiol in a dose-dependent fashion. Myoblast proliferation, however, as measured by BrdU incorporation, was induced by estrone but not 17-β estradiol. Further, investigators determined whether estrone or 17-β estradiol can induce expression of the immediate early genes egr-1 and c-fos. Treatment of myoblasts with 17-β estradiol for 30 min resulted in a 1.7-fold increase in c-fos and a 2.3-fold increase in egr-1 expression. Treatment of myoblasts with estrone for 30 min led to a 3.9- and 4.6-fold increase, respectively, in c-fox and egr-1. Treatment of cells with ICI 182,780 abolished responses indicating that E2 effects were mediated by the ER. More study is needed to understand why both forms of E2 induced the transcription factors egr-1 and c-fos but did not have similar effects on myoblast proliferation.
To further establish a role for E2 in skeletal muscle cell growth (myogenesis), Galuzzo et al. [27] studied the response of L6 cells to E2. Specifically, they examined ER-mediated nuclear signal transduction pathways. Briefly, they determined that E2 increased myogenin and myosin heavy chain (MHC) levels. Further, when they added the ER antagonist ICI 182,780 to the culture medium, myogenin and MHC expression failed to occur, indicating that the E2 effects on muscle cell growth were mediated by the ER. E2 also induced phosphorylation of p38 which is required for the expression of myogenin and MHC, and is crucial for transcriptional control of skeletal muscle differentiation. Interestingly, when these investigators added the extranuclear ER blocker 2-bromopalmitate, myogenin and MHC expression failed to occur. Thus, data indicate that E2-dependent rapid signaling from the membrane and nuclear action are responsible for the induction of L6 differentiation. These results need further elaboration.
Human skeletal muscle cells (myoblasts) were cultured by Dieli-Conwright et al. [29] and treated with E2. RT-PCR was used to determine the expression levels of mRNAs for steroid receptor coactivator (SRC), a positive regulator of ER activity, and silencing mediator for retinoid and thyroid hormone receptors (SMRT), a negative regulator of ER activity. E2 treatment for 24 h resulted in increased mRNA expression of SRC and decreased expression of SMRT. Additionally, E2 resulted in increased mRNA expression for MyoD, a potent stimulator of myoblast differentiation. Results suggest a role of E2 in the maintenance of skeletal muscle mass and function and have implications for aging women.
These important studies strongly indicate a role of E2 as a hormone impacting skeletal muscle cell growth and may explain why women who are postmenopausal lose strength and muscle mass at an accelerated rate. Given the number of younger women who are premenopausal undergoing oophorectomy each year coupled with the enormous number of women with breast cancer who are receiving drugs to reduce E2 levels, there may be an enormous number of women of all ages who are strength deficient.
Indirect Effects of E2 on Skeletal Muscle
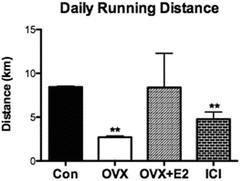
E2 exerts a powerful behavioral effect on spontaneous physical activity. Rodent studies are particularly dramatic in that if ovarian hormones are removed (OVX), spontaneous physical activity (wheel running) plummets almost immediately. Indeed, distances are 20–25 % of those prior to OVX. When the ovarian hormone E2 is provided back to the organism, running distances return to pre-OVX levels (Fig. 3.3). In our lab mature 4-month-old mice were placed in cages with running wheels and distances traveled were recorded daily for 6 weeks. Subsequently an OVX was performed and mice were returned to their cages with running wheels and followed another 3 weeks. With OVX running distances plummeted to values that were approximately 20 % of those recorded when ovaries were functioning normally. After the 3-week recording period following OVX, E2 was then provided to confirm that it was the ovarian hormone influencing spontaneous activity. Running distances returned to baseline levels, those recorded before OVX.