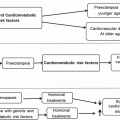
Fig. 9.1
Diagram summarizes known effects of aging and estrogen on HSPs and inflammatory stimuli in the heart. Dashed line—E2 effects; solid line—aging effects; arrow—stimulated; blunted line—inhibition
Although estrogen loss predominantly occurs later in life, basic studies on estrogen overwhelmingly have focused on young models such as 6–8-week-old mice. Because of this dearth of studies in aged models, studies on younger models will be discussed as well as those on aged models. This does not imply that there are no differences with aging, rather that much more investigation in aged models is needed.
Aging, Estrogen, and Cardiac Function—It has been unclear if aging and loss of estrogen results in a decline in cardiac function. Subtle changes in cardiac function were reported in postmenopausal women compared to age-matched controls [5]. However, the mean age of these women was only 47. A careful study of cardiac function in intact Fischer 344 rats measured cardiac functional parameters at 4, 13, 22, and 30 months of age [6]. Fractional shortening decreased significantly at 22 and 30 months from 54.8 ± 8 % to 47 ∀ 7 at 22 months and 43 ∀ 9 at 30 months. Estrogen levels were not measured in this study, but levels are known to decline with age in the rat. Investigation of diastolic function demonstrated that isovolumic relaxation time increased by 30 % at 30 months [6]. At the same time there was evidence of LV hypertrophy and increased LV volume with advancing age. Others have reported a drop in fractional shortening in aged ovariectomized Norway Brown (NB) rats at 22 months compared to ovariectomized rats treated with 17β-estradiol (E2, the most potent human estrogen) replacement [7]. Studies of isolated cardiac myocytes from 10-week-old SD rats 10 weeks post ovariectomy (ovx), with half receiving E2 replacement, found that the ovx myocytes had decreased +dL/dt compared to sham surgery and ovx + E2 replacement [8]. Multiple other parameters were abnormal in the ovx group including peak shortening and −dL/dt. Calcium handling was also impaired with increased resting calcium in the ovx group. This was accompanied by a reduced SERCA2a/phospholamban ratio in the ovx group [8]. Thus, several studies support loss of cardiac function with aging and estrogen loss and this has implications for the aging population.
Cardiac Myocyte Changes with Aging
Clinical trials, although very important, are limited in their ability to provide insights into underlying mechanisms. Thus, basic studies on appropriate models, such as the aging vs. adult rodent with and without estrogen, are important for understanding differences related to aging vs. estrogen loss, in order that we can better elucidate the mechanisms of cardiovascular changes in aging females. Unfortunately, work in this area is very limited. Basic studies on cardiac myocytes from aged and adult (22 vs. 6 months) ovx NB rats with and without immediate E2 replacement demonstrated disparate responses with aging and estrogen loss [7]. In the intact rats, serum inflammatory cytokines, interleukin (IL)-6, and tumor necrosis factor alpha (TNF) did not differ among the adult and aged rats with and without E2 [7]. Cultured cardiac myocytes derived from the aged ovx rats had much higher expression of IL-6 and TNF at the mRNA level. This increase in IL-6 and TNF could be attenuated by treatment with E2 for 6 h. Furthermore, the aged ovx cardiac myocytes had a greatly impaired ability to handle reactive oxygen species (ROS), the production of which is known to increase with age [9, 10]. E2 treatment in culture had no effect on the inability of the aged ovx cardiac myocytes to handle ROS. Expression of antioxidant genes did not differ between young and aged groups. Thus for the aged cardiac myocytes there was an increase in inflammatory cytokine expression and a markedly reduced ability to handle ROS with aging and loss of estrogen (Fig. 9.1). The results suggest that tissue levels of cytokines may be more revealing than plasma levels. In vivo E2 replacement improved many of these changes, and cytokine expression in culture was blunted by the addition of E2 to the media.
The Heat Shock Response, Aging, Estrogen, and Cardiac Protection
The heat shock response is a protective response that occurs with a diverse set of cell and tissue injuries and stresses including ischemia, hypoxia/reoxygenation, stretch (including angioplasty), and heat [11–13]. The heat shock response was originally described in heat-shocked drosophila in the 1960s. Heat and other stresses led to activation of heat shock factors (HSF), the predominant one for the heat shock response being HSF1. HSF1 is phosphorylated, trimerizes, and moves to the nucleus where it binds to heat shock elements in the promoters of the heat shock protein (HSP) genes, stimulating their transcription and translation. HSP72, the inducible HSP70, increases several fold with ischemia/reperfusion as well as with simulated ischemia in isolated adult cardiac myocytes, and inhibition of the increase in HSP72 with antisense, increases cell injury [14–16]. Thus, the endogenous heat shock response is protective. Further studies have shown convincingly that over expression of HSP72 in the heart protects against ischemic injury [17–19]. Thus, the heat shock response is vital to protection of the heart from injury.
E2 indirectly regulates cardiac HSP 72 expression in both male and female adult rat cardiac myocytes [20–22]. Cardiac HSP72 levels in female Sprague Dawley (SD) rats are twice the levels of males [22]. Ovariectomy leads to a drop in cardiac levels of female SD rats, but this change takes 9 weeks, suggesting that estrogen indirectly regulates the expression of HSP72 and that loss of estrogen leads to a cascade of changes. This finding has implications for the timing of studies looking at changes post ovariectomy. E2 replacement at the time of ovariectomy prevented the drop in HSP72 [22]. In addition to its role in protein folding, HSP72 has cardioprotective effects in transgenic models, reduces apoptosis by stabilizing the mitochondrial membrane, and prevents apoptosome formation [18, 23–25]. In isolated adult SD female cardiomyocytes, E2 treatment increased HSP 72 expression through the consecutive activation of the transcription factors, NFκB and HSF1 [26]. E2 pretreatment also protected against hypoxia/reoxygenation in cell culture [20, 27]. Recently we have shown that E2 activates NFκB via simultaneous activation of Akt, P38, and JNK followed by activation of ERK 1/2 and that inhibition of Akt. P38 or JNK prevents activation of NFκB [28]. Two synthetic estrogen receptor modulators, tamoxifen and raloxifene, also rapidly activated NFκB, but neither of these SERMs activated JNK [28]. Both tamoxifen and raloxifene mediated activation of NFκB via Akt and P38 leading to ERK 1/2 activation and cardiac myocyte protection. However, NFκB activation by each of these SERMs was less than that seen with E2. Although E2 can induce cardiac HSP 72 expression in adults, little is known how the loss of estrogen that occurs naturally during menopause in aging female rats affects the cardiac HSP response and the adaptive response to stress.
HSPs, the Heat Shock Response and the Aging Heart—The response of the aged heart to estrogen differs from the younger heart. Cardiac myocytes derived from aged (22 months) and adult (6 months) NB rats 9 weeks post ovx with and without immediate E2 replacement were studied to determine the role of E2 in ameliorating the inflammatory changes of aging. The adult cardiac myocytes, regardless of E2 replacement status, showed activation of NFκB and increased HSP72 expression with estrogen treatment in culture [7]. However, neither aged group had NFκB nor increased HSP72 in response to E2, and in fact NFκB was activated at baseline in the aged ovx cardiac myocytes. Hypoxia and reoxygenation induce the heat shock response with activation of HSF1 and increased HSP expression, a protective response. As expected, both groups of adult cardiac myocytes had activation of the heat shock response, but the aged cardiac myocytes regardless of estrogen replacement had no activation of HSF1 and no increase in HSP72 [7]. HSF1 expression levels did not differ among the groups, but in the aged ovx phosphorylation at serines 303/307, which inhibits activation of transcription, but not binding to the promoter by HSF1, was present [7]. Thus with aging, in the female heart there was inhibition of the protective heat shock response secondary to posttranslational modification of HSF1. Estrogen replacement had no effect on this response. Similar loss of the heat shock response and loss of activation of HSF1 have also been found in male models of aging with different mechanisms proposed [29–31]. One mechanism of inhibition of HSF1 is acetylation of the transcription factor at lysine 80, which prevents activation of HSF1 by preventing binding of HSF1 to the heat shock element [32]. In the aged female rat cardiac myocytes, there was no evidence of acetylation of HSF1 [7]. These differences in mechanisms of inactivation of HSF1 in aging female cardiac myocytes and other tissue types and cell lines may reflect that there are multiple mechanisms regulating HSF1 activation or true gender/aging/tissue differences. Given the recent interesting link of DNA-damage-associated cell senescence with a drop in HSF1 as well as the importance of loss of the heat shock response in aging, clearly more investigation is warranted in this area [33, 34].
Ischemia and other injuries induce activation of the heat shock response with activation of HSF1 and increased expression of HSPs, particularly HSP72. Simulated ischemia failed to induce activation of HSF1 and increased expression of the cardioprotective heat shock protein (HSP)72 in aged female cardiac myocytes from aged ovariectomized NB rats, regardless of the presence or absence of estrogen replacement. In contrast, cardiac myocytes from ovariectomized adult NB rats had HSF1 activation and increased HSP72 with simulated ischemia regardless of estrogen status. In the aged cardiac myocytes only, regardless of E2 replacement, HSF1 was inactivated by phosphorylation at serines 303/307, and there was no increase in the cardioprotective HSPs after simulated ischemia [7]. As discussed above, E2 replacement in vivo prevented many of the increase in cytokines and ROS with aging and OVX, and in vitro addition of E2 to aged ovx-cultured cardiac myocytes reduced cytokine expression; however, neither in vivo nor in vitro E2 replacement prevented the inactivation of HSF1 associated with aging. This has important implications for patients, as ROS and inflammatory cytokines, such as TNF, are an important causes of cardiac injury. The heat shock response mediated by activation of HSF1 is important for cardiac protection, and loss of the heat shock response makes the aging heart more vulnerable to injury.
E2 and Mitochondrial Protection
The mitochondrion, despite its importance for the generation of high energy phosphates to provide energy to fuel contraction, is also an important contributor to cardiac injury. Mitochondria produce a large amount of ROS as a by-product of energy production. In normal tissue, there is an extensive system of antioxidants and scavengers to prevent damage from these ROS. However, ischemic injury alters the balance of antioxidants and ROS. E2 is cardioprotective in the setting of ischemic injury, and amelioration of mitochondrial damage is likely a key component of this protective response. E2 treatment inhibited cytochrome c release from isolated cardiac mitochondria exposed to high calcium levels [35]. Likewise, E2 prevented cytochrome c release in global ischemia in the isolated perfused heart; furthermore E2 protected mitochondrial respiration and inhibited DNA fragmentation [36]. In isolated neonatal myocytes subjected to hypoxia and reoxygenation E2 reduced apoptosis and ameliorated ROS production mediated by prevention of p53 phosphorylation and translocation to the mitochondria [37]. In the isolated perfused heart the protective effects of E2(100 nM) during global ischemia/reperfusion were markedly decreased by inhibition of protein kinase G [38]. Similarly, inhibition of either Akt or eNOS reduced E2’s protective effects [39]. E2 was also protective in trauma-hemorrhage (T-H), and this protection was mediated by ERβ, which lessened the reduction in cardiac mitochondrial ATP, inhibited lipid accumulation, and protected protein expression [40]. Inhibition of PGC-1α blocked E2’s protective effects in a Tfam-dependent manner [40]. Thus protection of the mitochondria and reduction of ROS production are critical protective effects of E2 (Fig. 9.1).
Mitochondrial Proteomic Changes—E2 influenced the mitochondrial phospho-protein profile in young rat hearts [41]. Aldehyde dehydrogenase 2 (ALDH2), which detoxifies ROS, has increased phosphorylation and increased activity in the female heart [41]. This increase in ALDH2 phosphorylation and activity is blocked by Wortmannin treatment. α-Ketoglutarate dehydrogenase (αKDGH), which is a major source of ROS with high NADH/NAD+ ratios, as found with ischemia/reperfusion, also had increased phosphorylation, but evidence suggests the increased phosphorylation may decrease activity and thus decrease ROS [41]. Whether these effects would occur in aged mitochondria is unknown.
Aging, Estrogen, and Mitochondrial Protection—Studies in aged hearts on estrogen and mitochondrial function have been quite limited. Aging, regardless of estrogen status, was associated with a small decrease in the respiratory control index (RCI, state 3/state 2) for complexes I and II [42]. Pretreatment with the selective estrogen agonist, propyl pyrazole triol (PPT), increased RCI for complexes I and II, but also increased Ca2+ sensitivity in aged female mitochondria, regardless of estrogen status, suggesting ER signaling could be a double-edged sword in the aged mitochondria. [42]
Estrogen, Ischemia, and Cardiac Protection
There is a substantial literature demonstrating that estrogen can protect the heart from ischemic injury and several first-rate reviews have recently addressed this topic [43–45]. Activation of either ERα or ERβ has been shown to protect the heart from ischemia/reperfusion injury using knockout models as well as selective agonists [43, 46–49]. More recently a third, membrane associated ER, G-protein-coupled estrogen receptor (GPER) has been identified [50]. Pretreatment with G-1, a selective GPER agonist, decreased infarct size and enhanced functional recovery in a Langendorff perfused-heart model. Like ERα, GPER activated both Akt and ERK 1/2 [51]. Although some disagreement persists, the literature supports that ERα and ERβ each can activate PI3K, Akt, and a anti-apoptotic, protective signaling cascade [47].
Estrogen and Protection in Trauma Hemorrhage—A number of studies have investigated the benefit of E2 treatment in models of trauma hemorrhage (T-H) and neural injury. In T-H models reduction in organ neutrophil infiltration and improved cardiac function with a single treatment of E2 have been found [52, 53]. A one time treatment with high dose of E2 reduced mortality and improved recovery in a rat model of trauma [54]. A wide array of endpoints are improved with E2 treatment in basic studies of trauma hemorrhage and changes associated with E2 treatment included reduction in inflammatory cytokine levels and enhanced expression of heat shock proteins [55]. AKT activation by E2 led to increased heme oxygenase (HO)-1 expression in trauma hemorrhage [56]. Likewise, others have reported that female rats at peak estrus were had improved morbidity and mortality after trauma [57]. Similarly, female rats in proestrus/estrus, when estrogen levels would be high, had less red blood cell damage and less lipid peroxidation compared to males and low estrogen females after T-H-hemorrhage [58]. E2 just prior to cerebral ischemia increased heat shock protein expression in brain arteries, as well as glia and neurons [58]. Others found no difference in a spinal cord contusion model between females at low and high estrogen levels [59]; however, these studies were based on vaginal smears as an indicator of estrogen levels and compared proestrus and estrus female rats. Depending on the exact hour given the 4-day rat cycle, there may not have been striking differences in the estrogen levels, which were not measured, between groups. In a prospective clinical study of polytrauma, female patients less than 50, suggesting the presence of estrogen, have been shown to have better outcomes and less complications, including less days with multiorgan dysfunction syndrome (MODS), lower cytokine levels and less sepsis than age-matched males [60]. A weakness of the study was the assumption that women 50 or younger were premenopausal and estrogen levels were not measured. However, overall the studies on trauma-hemorrhage are intriguing and raise the possibility that the benefit of a single high dose of E2 in the laboratory can be applied to trauma victims in the emergency room.
Heart Failure
Systolic Heart Failure—The prevalence of systolic heart failure (sHF) has increased over the last 10–15 years as a result of improvements in the treatment of acute heart disease. SHF, with dilation of the ventricle and loss of systolic function, occurs as a result of coronary disease and myocardial infarction (ischemic heart failure) as well as secondary to a diverse set of causes such as toxins and viruses, which are referred to as dilated cardiomyopathy or more recently as nonischemic cardiomyopathy. Gender differences have been reported in systolic heart failure characterized by an improved course and better survival in females [61, 62]. A retrospective analysis conducted using the Vesnarinone database found better survival in women taking estrogen. In contrast, analysis of the CHARM database demonstrated improved survival for women, but the presence or absence of HRT had no effect on survival [61, 63]. Given that there can be significant variation in the drugs used in HRT, it is not surprising that no difference was observed. Other studies have reported no gender difference in heart failure survival [64]. The pooled analysis of five heart failure trials again suggested longer survival for female patients [65]. Overall, the trial data suggests a possible gender variation, but underlying weaknesses preclude the drawing of strong conclusions. For some of the studies follow-up was only a year, too short to discern survival differences. All of these papers are retrospective analyses of databases collected with other aims, the number of female patients is limited and only two databases had data on the use of estrogen. Another issue is that gender and estrogen are used interchangeably for many of these studies, but women with heart failure may be premenopausal, postmenopausal, or postmenopausal on HRT. Thus these patients may have widely varying estrogen levels. Ergo, comparing the outcomes for all female patients vs. all males will be misleading unless the estrogen status of the female population is established. The type of estrogen replacement is also important, and benefit might not be seen with conjugated equine estrogen (CEE), which has been the most commonly used source of estrogen for HRT. CEE is derived from the urine of pregnant mares and contains no 17β-estradiol, which is the most potent form of estrogen found in women [44]. The type of estrogen(s) present matters as different estrogens will have different binding affinities and different selectivity for the estrogen receptors. CEE does contain estrones, and estrone levels have been linked to the generation of thrombin, which is a key step in the activation of coagulation [66]. Furthermore, most HRT has been via oral replacement and this leads to high hepatic concentrations of estrogens, secondary to first pass metabolism. High hepatic estrogen levels will alter gene expression, as a major function of ERα and ERβ is as transcription factors, and may increase the expression of proteins involved in the clotting cascade. Transdermal HRT is thought to be safer as it avoids the high hepatic levels of estrogen seen with oral replacement. The heart failure databases contain little or no data on actual estrogen therapy, as the original intent of these studies was not focused on the effects of estrogen.
Overall the data on gender, estrogen, and morbidity and mortality is insufficient to draw any definitive conclusions with regards to a gender benefit or estrogen benefit in heart failure. Certainly there are many basic studies suggesting that estrogen has protective effects in the heart. Clinical studies directly investigating the effects of gender and/or HRT are needed to determine whether estrogen or gender improve survival in heart failure. The development of synthetic estrogen receptor modulators (SERMs, discussed in more detail below) raises the possibility of having a gender neutral estrogen analogue that can activate protective responses in the heart as E2 does.
A number of more focused studies have reported gender benefits in sHF. For example, female patients with sHF show greater benefit with cardiac resynchronization therapy (CRT) than male patients, but whether this is a gender-based difference or hormonal, remains unknown [67]. Explanted failing hearts removed during heart transplants have been extensively studied. It is known that the rate of apoptosis is increased in the failing heart, but in addition it has been found the end-stage failing female hearts have half the rate of cell death as male hearts [68]. Others have identified some differences in cardiac gene expression between females and males with new onset heart failure [69]. Females had higher expression PDE6b, which is cardioprotective, GLUT12, involved in glucose transport, and GATAD1, which is involved in the regulation of adrenergic and angiotensin receptor trafficking. Males had higher expression of CD24, which is involved in regulation of immunity.
Basic investigations on gender and heart failure have been quite limited. ERα is nearly double in nonischemic cardiomyopathy independent of gender [70]. In the normal heart ERα is found in the cytosol, sarcolemma, intercalated discs, and nuclei. In contrast, in end-stage failing hearts, neither ERα or connexin 43 was present in the intercalated discs. The Knaub group has made the interesting observation that in heart failure cyclic nucleotide regulatory binding protein (CREB) was disproportionately downregulated in female hearts. Female hearts were very susceptible to downregulation of CREB with a dominant negative transgene construct, while male littermates showed little response to this reduction in CREB [71]. As early as 4-week female hearts had increased ROS, decreased MnSOD, and decreased glutathione peroxidase. These changes were accompanied by loss of mitochondrial cristae, decreased cardiac function, and markedly increased mortality by 21 weeks. In contrast, males had far less evidence of cellular injury and much greater survival. CREB has been found to decrease in heart failure, it is possible that this decrease in CREB makes the course of heart failure more severe in females, though there is uncertainty as to whether females have better or worse survival in heart failure, as discussed above, and many questions remain to be answered [71].
Diastolic Heart Failure—Approximately half of heart failure is diastolic heart failure (dHF), which in contrast to sHF, is characterized by preservation of ejection fraction but impaired relaxation. Diastolic heart failure occurs secondary to pathologic hypertrophy, most commonly occurring as a result of hypertension [72, 73]. In pathologic hypertrophy there is inadequate capillary proliferation, leading to decreased capillary density and fibrosis. On the other hand, normal/physiologic hypertrophy occurs in response to exercise, pregnancy, and normal growth. Physiologic hypertrophy has normal capillary density without fibrosis. DHF is thought more prevalent in women and the incidence increases with aging. Therefore, women with dHF will tend to be postmenopausal and likely not on HRT, as HRT use has dropped dramatically in the last 10 years [74, 75]. Abnormal diastolic relaxation results in an increased left ventricular end-diastolic pressure, and this leads to elevated pressure in the pulmonary vasculature, which can lead to pulmonary congestion. Hence, despite good cardiac contractility, patients with dHF become dyspneic. Notwithstanding the preservation of ejection fraction, diastolic heart failure has a prognosis that is as grim as that for systolic heart failure. Investigators at the Mayo Clinic reported a 50 % 5-year survival in patients with dHF [76]. DHF can be more difficult to manage than sHF, where multiple medications are available to reduce symptoms and prolong survival. Treatment for dHF relies on beta blockers and calcium channel blockers to reduce the heart rate and possibly improve diastolic relaxation. In addition diuretics are used to reduce breathlessness. There have been no advances in treatment of diastolic heart failure in many years, and the effectiveness of current treatment is quite unsatisfactory.
Estrogen and Pathologic Cardiac Hypertrophy
Cardiac hypertrophy is more common in males. Transverse aortic constriction (TAC) has frequently been used as a model to investigate the effects of gender, E2 and ERα and ERβ on cardiac hypertrophy. WT, ERα, and ERβ knockout mice were ovariectomized and half were begun on standard sustained-release E2 replacement. E2 replacement reduced TAC-induced cardiac hypertrophy in ERα knockout but not in ERβ knockout mice [77]. E2-mediated reduction in TAC-associated hypertrophy was not associated with a change in cardiac function. In contrast, uterine hypertrophy was inhibited by ERα knockout but not by ERβ knockout. In ER∀ knockout and WT mice, but not ERβ knockout mice, E2 replacement prevented increased phosphorylation of p38, increased ANP, and decreased hypertrophy. In studies of intact female mice compared to males, males had more hypertrophy and heart failure post TAC. TAC led to increased matrix and mitochondrial gene expression, and again the changes were more pronounced in males than females. After ER∃ knockout, greater hypertrophy, including increased cardiac myocyte diameter ensued in both males and females [78]. ERβ knockout mice had increased expression of pro-apoptotic genes, and this was more marked in males than females. These studies are consistent with ERβ mediating the protective effects of E2 in pathologic hypertrophy.
Some very intriguing work has been done linking estrogen to increased calcineurin degradation, and this is mediated by ER receptor pathway, and is possibly specific to ERβ [79, 80]. E2 signaling promotes increased degradation of calcineurin via ubiquitination and the 26 s proteasomal system. In calcineurin knockout mice, E2 treatment did not inhibit LV hypertrophy [80]. Calcineurin knockout prevents downstream signaling by the NFAT family of transcription factors, which are key targets of calcineurin. These findings suggest that calcineurin degradation may be a major pathway for E2-mediated inhibition of hypertrophy. This work again implicates ER∃ as critical in mediating the protective responses to E2 in cardiac hypertrophy.
Quite interesting basic investigation has shown that E2 can modulate the pathologic hypertrophic response through several other mechanisms. There are three known estrogen receptors: ERα, ERβ, and GPER (also known as GPR30). In models of pathologic hypertrophy, E2 suppressed MMP2 expression, fibrosis, and hypertrophy through activation of ERβ [77–79, 81–83]. Nevertheless, the typical model for much of this work is 6–8-week-old mice, and these findings may not translate to older models or to aging humans. Others have demonstrated that GPER activation attenuates diastolic dysfunction as well as ventricular remodeling post ovariectomy in an aging hypertensive rat model [84]. These findings are very promising as GPER activation is not known to be associated with the adverse effects of estrogen, and dHF remains a difficult problem. New insights into the underlying mechanisms leading to dHF are needed to help develop better therapies. The use of aged models, to determine if E2 has similar effects in aging, is essential to develop new therapies for dHF.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree