Primary tumor (T)*
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
High-grade dysplasia**
T1
Tumor invades lamina propria, muscularis mucosae, or submucosa
T1a
Tumor invades lamina propria or muscularis mucosae
T1b
Tumor invades submucosa
T2
Tumor invades muscularis propria
T3
Tumor invades adventitia
T4
Tumor invades adjacent structures
T4a
Resectable tumor invading pleura, pericardium, or diaphragm
T4b
Unresectable tumor invading other adjacent structures such as aorta, vertebral body, trachea, etc.
*At least maximal dimension of the tumor must be recorded and multiple tumors require the T(m) suffix
**High-grade dysplasia includes all noninvasive neoplastic epithelial that was formerly called carcinoma in situ, a diagnosis that is no longer used for columnar mucosae anywhere in the GIT
Regional lymph nodes (N)* | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph nodes metastasis |
N1 | Metastasis in 1–2 regional lymph nodes |
N2 | Metastasis in 3–6 regional lymph nodes |
N3 | Metastasis in ≥7 regional lymph nodes |
*Number must be recorded for the total number of regional nodes sampled and total number of reported with metastases | |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Adapted with permission from AJCC: Esophagus and Esophagogastric Junction. In: Compton C, Byrd D, Garcia-Aguilar J, Kurtzman S, Olawaiye A, Washington M, eds.: AJCC Cancer Staging Atlas. 2nd ed. New York, NY: Springer, 2012, pp 129 | |
Anatomic stage/prognostic groups | |||||
|---|---|---|---|---|---|
Squamous cell carcinoma* | |||||
Stage | T | N | M | Grade | Tumor location** |
0 | Tis (HGD) | N0 | M0 | 1, X | Any |
IA | T1 | N0 | M0 | 1, X | Any |
IB | T1 | N0 | M0 | 2–3 | Any |
T2-3 | N0 | M0 | 1, X | Lower, X | |
IIA | T2-3 | N0 | M0 | 1, X | Upper, middle |
T2-3 | N0 | M0 | 2–3 | Lower, X | |
IIB | T2-3 | N0 | M0 | 2–3 | Upper, middle |
T1-2 | N1 | M0 | Any | Any | |
IIIA | T1-2 | N2 | M0 | Any | Any |
T3 | N1 | M0 | Any | Any | |
T4a | N0 | M0 | Any | Any | |
IIIB | T3 | N2 | M0 | Any | Any |
IIIC | T4a | N1-2 | M0 | Any | Any |
T4b | Any | M0 | Any | Any | |
Any | N3 | M0 | Any | Any | |
IV | Any | Any | M1 | Any | Any |
*Or mixed histology including a squamous component or NOS | |||||
**Location of primary cancer site is defined by the position of the upper (proximal) edge of the tumor in the esophagus | |||||
Adenocarcinoma | ||||
|---|---|---|---|---|
Stage | T | N | M | Grade |
0 | Tis (HGD) | N0 | M0 | 1, X |
IA | T1 | N0 | M0 | 1–2, X |
IB | T1 | N0 | M0 | 3 |
T2 | N0 | M0 | 1–2, X | |
IIA | T2 | N0 | M0 | 3 |
IIB | T3 | N0 | M0 | Any |
T1-2 | N1 | M0 | Any | |
IIIA | T1-2 | N2 | M0 | Any |
T3 | N1 | M0 | Any | |
T4a | N0 | M0 | Any | |
IIIB | T3 | N2 | M0 | Any |
IIIC | T4a | N1-2 | M0 | Any |
T4b | Any | M0 | Any | |
Any | N3 | M0 | Any | |
IV | Any | Any | M1 | Any |
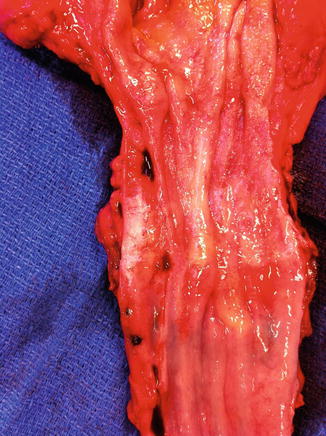
Fig. 8.1
An early stage distal esophageal adenocarcinoma contained within the adventitia
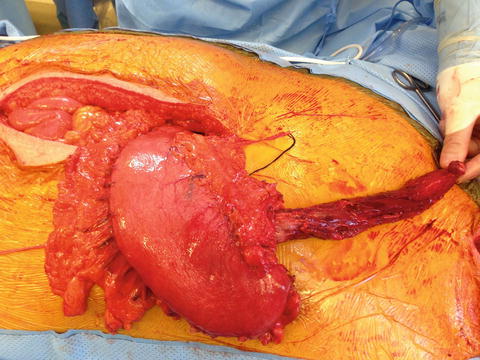
Fig. 8.2
A locally advanced gastroesophageal junction lesion with involvement of the lesser curvature of the stomach
The overall survival by the previous staging system (as long-term follow up is currently unavailable for the current staging system) is approximately as follows: [22]
Stage 0: 100 %
Stage I: 30–40 %
Stage IIA: 30–40 %
Stage IIB: 10–30 %
Stage III: 10–15 %
Stage IV: 0–5 %
Overall prognosis can also be thought in terms of localized, regional, or distant disease with 5-year relative survival rates of 37 %, 18 %, and 3 %, respectively [23].
Clinical Presentation
The most common symptoms on presentation include progressive dysphagia from solids to liquids and weight loss. These often come on slow and indolently, and patients often present late in their disease. Association of symptoms with more locally advanced disease includes hoarseness from recurrent laryngeal nerve involvement, chest pain from thoracic and mediastinal invasion, back pain from vertebral body invasion, and dyspnea from tracheoesophageal or bronchoesophageal fistula formation.
Workup
Historically, patients received a barium swallow with plain films (esophagography) to detect the presence of an esophageal mass. Nowadays, additional confirmatory tests include esophagogastroduodenoscopy (EGD) for direct visualization of the upper GI tract and determination of the extent of longitudinal spread and biopsy confirmation (Fig. 8.3). This is then followed by an endoscopic ultrasound (EUS) with a fine needle aspiration (FNA). EUS is the ideal imaging modality to assess depth of tumor invasion with a T-stage sensitivity of 81–90 % and specificity of about 99 % and an N-stage sensitivity of 96.7 % and specificity of 95.5 % with FNA [24]. Additional tests include a computed tomography (CT) scan and an integrated positron emission tomography and CT scan (PET/CT).
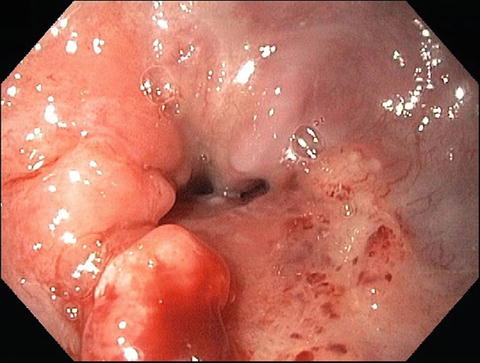

Fig. 8.3
An endoscopic picture of a distal esophageal adenocarcinoma (Courtesy of Quyen D. Chu, MD, MBA, FACS)
CT scans allows anatomic visualization of the extent of spread, lymphadenopathy, and relations with adjacent structures that assists surgeons in determining feasibility and extent of resection, as well as assists radiation oncologists in determining the extent of their treatment fields. CT detection of nodal metastases has an accuracy rate of 68–96 %, sensitivity rate of 8–75 %, and specificity rate of 77–94 % [25]. These rates are variable because they depend on the location of the nodal groups. For example, the sensitivity rate for detecting cervical paraesophageal nodes is 75 %, whereas it is 8 % for lesser curvature nodes.
PET/CT scans allow the visualization of metabolic activity to enhance identification of distant metastases and can change patient management up to 20 % of the cases [26]. PET/CT sensitivity to detect locoregional metastases is 51 % with a specificity of 84 %, and its sensitivity for detecting distant metastases is 67 % with a specificity of 97 % [26]. Pre- and post-treatment standardized uptake values (SUV) from PET may also have prognostic significance [27]. Additional workup for tumors above the carina without evidence of metastatic disease includes bronchoscopy and optional laparoscopy for GEJ tumors without evidence of metastatic disease [9].
Treatment
A multimodality approach is essential for the treatment of esophageal cancer, as treatment options vary widely according to stage. Table 8.2 demonstrates basic outline of treatment recommendations.
Table 8.2
Treatment recommendations by stage
Stage | Treatment |
|---|---|
Tis–T1a | Endoscopic mucosal resection, photodynamic therapy, radiofrequency ablation, or esophagectomy alone |
T1b | Esophagectomy alone |
T2–T4, node positive | Neoadjuvant chemoradiation followed by esophagectomy |
Distant metastasis | Palliative chemotherapy, radiation, or chemoradiation, esophageal dilation and stenting |
For carcinoma in situ (high-grade dysplasia) or lesions limited to the lamina propria and muscularis mucosa (Tis–T1a), a superficial approach is an option since these lesions have a low risk of spread and a cancer mortality of only 5 %. However, invasion into the submucosa dramatically increases the risk of spread and has a mortality risk of 40 %; hence superficial treatments are recommended only for stages Tis–T1a.
The three main superficial approaches include endoscopic mucosal resection (EMR), photodynamic therapy (PDT), and radiofrequency ablation (RFA). EMR involves a submucosal injection of fluid to separate the lesion from the muscular layer of the esophagus to allow complete resection of the lesion [2]. Photodynamic therapy (PDT) uses monochromatic light to excite a photosensitizing agent that is selectively concentrated into malignant tissues causing the production of superoxide and hydroxyl radicals that lead to apoptosis, necrosis, vascular occlusion, and activation of the immune response [28]. RFA involves the use of ablation catheters inside a cylindrical balloon that is placed adjacent to lesions to ablate tissues using heat via an electrical current [2, 11]. Since RFA heating requires an electrically conductive path, it results in heating of tissues just next to the catheter [29].
A study by the University of Pennsylvania found that the sensitivity, specificity, and positive and negative predictive values of preoperative EUS for submucosal invasion were 100 %, 94 %, 83 %, and 100 %, respectively [30]. An Italian study found lower rates of accuracy with sensitivity, specificity, and positive and negative predictive values of 88 %, 63 %, 67 %, and 86 %, respectively; however, the authors still conclude that EUS is an extremely useful tool when considering nonsurgical treatment options [31].
A prospective single institutional study was performed examining the role of EMR for mucosal lesions showing high-grade dysplasia or early cancer in Barrett’s esophagus after diagnosis by EUS [32]. EUS was able to accurately diagnose 85 % of the lesions (1 patient overstaged and 6 understaged). A meta-analysis of the staging accuracy of esophageal cancer by EUS found the sensitivity and specificity for T1 lesions to be 81.6 % and 99.4 %, respectively [24]. These studies provide further support that EMR is an acceptable treatment option after diagnosis by EUS. Nevertheless, the incidence of occult adenocarcinoma in high-grade dysplasia has been reported to be as high as 40 %, and if the lesion has characteristics that are worrisome for a predilection for lymph node metastases such as lymphovascular space invasion, neural invasion, tumor size > 2 cm, or multifocality, an esophagectomy, especially in the era of minimally invasive techniques, still warrants consideration [33–35]. The morbidity from such a procedure for early stage disease is acceptable as the University of Pittsburgh’s review of their T1 esophagectomy patients found that on their Gastroesophageal Reflux Disease-Health Related Quality of Life (GERD-HRQOL) questionnaire, 89 % and 10.6 % of patients had excellent and satisfactory HRQOL scores, respectively [35].
Surgery
After clinical staging is completed, treatment of esophageal cancers is relegated to three broad categories: immediate surgical therapy, surgical therapy following chemotherapy with or without radiation therapy, and nonsurgical management. Esophageal precancerous lesions such as high-grade dysplasia or very early cancers can be treated with the aforementioned strategies, but are also candidates for immediate resection. For the majority of esophageal cancers, however, advanced stage at diagnosis will mandate neoadjuvant treatment followed by surgical resection. In the lattermost category are the metastatic and unresectable cancers (i.e., those that locally invade vital structures such as the aorta or tracheobronchial tree) – these are not offered surgical resection, but rather offered palliative chemoradiotherapy. As the esophagus traverses both the thoracic and the abdominal cavities, there are understandably several approaches to facilitate its removal. Despite these variations, a few constants hold true which follow below.
In concordance with all other solid organ resections for malignancy, a minimum number of lymph nodes retrieved are recommended to constitute adequate diagnostic and therapeutic benefit. Based upon consensus statement by the National Comprehensive Cancer Network’s esophageal cancer work group, this number has been established at 15 nodes [36]. For upper and middle esophageal lesions, this mainly comprises the adventitial tissue surrounding the esophagus within the posterior mediastinum. For distal esophageal lesions, this represents paraesophageal nodes from the hiatal region and those along the course of the left gastric artery. In regard to surgical margins, obtaining a longitudinal esophageal margin is generally attainable considering the generous length of the organ; for upper third or low cervical tumors, however, this can sometimes require a partial or total laryngectomy with pharyngogastric reconstruction. It should be mentioned though that historically, due to poor function and survival outcomes, many experts feel that definitive chemoradiation therapy is more appropriate for proximal esophageal cancers.
The gastric margin, conversely, can become a concern in particular with lesions found at the gastroesophageal junction, which is an area frequently involved in western series [37]. In this situation, obtaining a comfortable margin of (preferably) five centimeters sometimes cannot be achieved while still preserving enough proximal stomach to fashion a suitable conduit for esophageal reconstruction (Figs. 8.4 and 8.5). In that instance, an alternate conduit is required such as colon or jejunum in selected circumstances (Fig. 8.6).
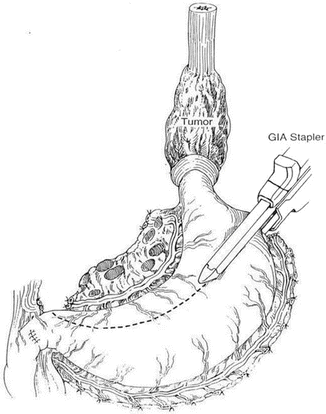
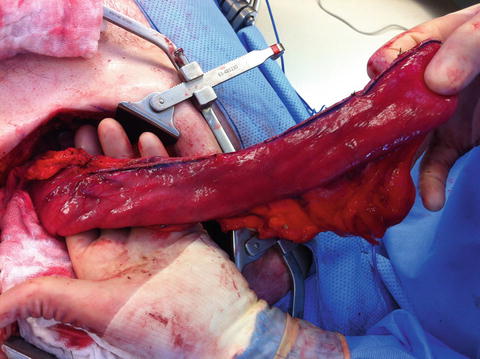
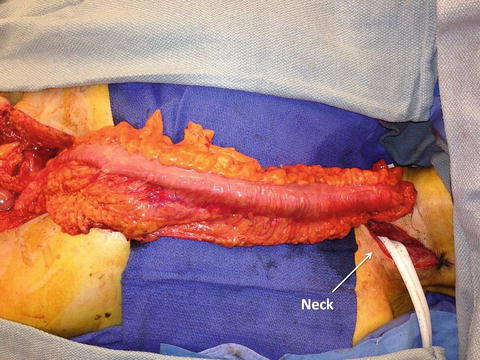

Fig. 8.4
Resecting the distal lesion of the esophagus and creating a gastric conduit. The stomach is mobilized after ligating all vessels supplying the stomach except for the right gastroepiploic vessel. Note that the lymph nodes along the lesser curvature are included in the specimen (Reprinted from Ref. [40]. With permission from Elsevier)

Fig. 8.5
A reconstructed esophagus using a gastric conduit

Fig. 8.6
Colonic interposition using the right colon. Blood supply is based on the inferior mesenteric artery (Courtesy of Quyen D. Chu, MD, MBA, FACS)
All patients undergoing esophageal resection will have several days of nil per os (NPO) status, and this period can increase substantially if there are any untoward complications such as anastomotic leak. Therefore, enteral access by routine placement of a jejunal feeding tube is highly recommended. This tube remains in place for roughly 1 month after surgery and is removed once the patient has demonstrated that adequate caloric intake and stabilization of postoperative weight loss can be achieved by oral diet alone. Another distinct advantage of a routine jejunostomy tube placement is that if the patient develops troubles with oral nutrition in the future, whether due to stricture, recurrence, or simply failure to thrive, the jejunostomy site can be percutaneously cannulated by an interventional radiologist and immediate enteral feedings can commence without difficulties [38]. In our practice, we have found this to be of great use even years after the initial surgery. For those patients who are plagued by continued weight loss or radiation-induced esophagitis preventing oral nutrition during the neoadjuvant phase of their treatment, it is sometimes necessary to have enteral access placed before esophagectomy. For these purposes, we have found either percutaneously placed gastrostomy tubes by interventional radiology or traditional surgical jejunostomy tube placement to be superior to percutaneous endoscopy gastrostomy (PEG) tubes. The benefit of the former preferred tube is that the footprint of a 14–16 French Cook-type catheter on the gastric wall is considerably smaller than the common 20–24 French PEG-style tube. A small catheter gastrostomy can be easily oversewn and imbricated frequently with one or two sutures, whereas the PEG style tubes often produce a more pronounced disfigurement of the gastric wall and can jeopardize the creation of a gastric tube.
Aside from these technical considerations of esophagectomy, there is an overarching concept that can differentiate overall success of surgical management: volume. In a recent meta-analysis comprising nearly 28,000 patients, the overall surgical mortality significantly decreased in proportion to the number of esophagectomy cases performed by the given institution per year [39]. Undoubtedly, as the expertise of the surgeon, surgical team, and postoperative care team increases, so does patient safety. One major aspect of this relationship is likely related to the resources available in high-volume centers in the arena of rescue therapies for postoperative complications. After a surgery of this magnitude, regardless of the approach, several significant complications can arise that require adjunctive procedures to remedy, such as percutaneous drain placements, complex endoscopic interventions, and sophisticated cardiopulmonary team care. Furthermore, the aptitude of experienced nursing personnel in identifying these potential complications in an early phase may allow more swift resolution before progression. In a center where only a small number of these cases occur annually, these resources may not have the level of expertise or readiness to rescue an ailing patient who is rapidly deteriorating.
Esophagectomy Techniques
Transhiatal
As its name implies, a transhiatal esophagectomy is constituted by dissecting the middle and distal thirds of the esophagus through the diaphragmatic hiatus without the need for thoracotomy (Fig. 8.7) [40]. A cervical exposure is used to dissect the proximal third and complete the dissection in continuity with the abdominal approach. A long gastric tube is created and then drawn through the posterior mediastinum until it reaches the skin level of the neck. An anastomosis between the cervical esophagus and this neo-esophagus is then created. Tumors located anywhere along the entire length of the esophagus to the gastric cardia can be resected equally with adequate margins. Advantages of this technique include the avoidance of entering the thorax, lower mortality from a leak at the cervical anastomosis, compared to a leak in the thorax from a transthoracic approach and the ability to remove the entire length of the esophagus when the consideration of long-segment Barrett’s or margins is of concern. Without a thoracotomy, the pulmonary complications often plaguing esophageal resection are generally mitigated, which has been substantiated in the literature by a decreased length of ICU stay for the transhiatal technique [41]. Cervical anastomoses are created outside the radiated field of the mediastinum, and leaks in this locale generally will fistulize to the cervical skin without even fever or abscess formation. Near uniform closure of these fistulas will follow with cessation of oral intake alone, in great contrast to the 4–10 % need for reoperation that follows a thoracic duct leak. Disadvantages to this technique include the limited ability to dissect middle esophageal tumors, a questionable completeness of radical lymphadenectomy in the posterior mediastinum, thoracic duct leak at the level of the left neck, higher anastomotic leak rate compared to the transthoracic approach, and the increased risk of inadvertent injury to the tracheobronchial tree or azygos vein during blunt dissection of the proximal esophagus. Additionally, in the absence of direct visualization, the recurrent laryngeal nerves are at increased risk of injury when compared to esophagectomy via thoracotomy. A jejunal feeding tube is placed in all patients to bolster nutritional intake.
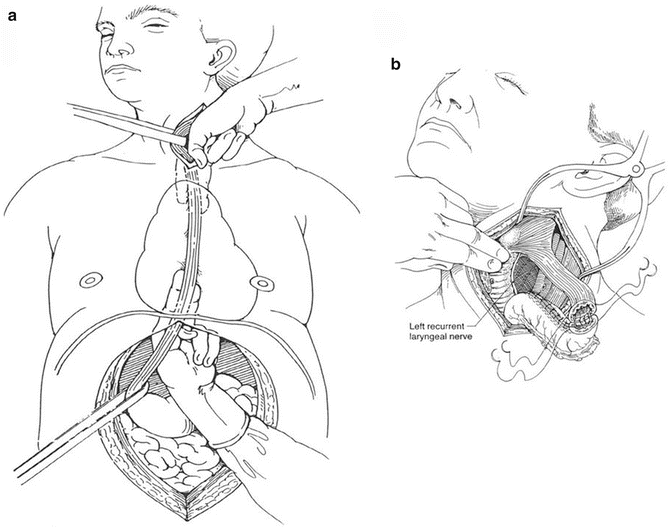

Fig. 8.7
Transhiatal esophagectomy technique. (a) The esophagus is bluntly dissected away from the surrounding structure with the fingers, taking care to not injure the membranous portion of the trachea. A left neck incision is also made to further mobilize the cervical esophagus. Care is taken to avoid injury to the left recurrent laryngeal nerve. (b) A cervical esophagogastrostomy anastomosis is performed in the left neck (Reprinted from Ref. [40]. With permission from Elsevier)
Transthoracic
Arguably the most commonly employed method of esophagectomy, resection via a right thoracotomy and laparotomy (Ivor-Lewis) remains the gold standard method of resecting any esophageal tumor of the middle and distal third as well as those extending to the gastric cardia [40, 42] (Fig. 8.8). Unlike a transhiatal approach, the advantage of direct dissection of the posterior mediastinum and the peri-esophageal tissue allows a more deliberate and thorough lymphadenectomy. The esophagus is divided at or above the level of the azygos vein, and a gastric tube similar to that created in the transhiatal procedure is anastomosed at that location. Advantages also include the ability to carefully preserve the membranous portions of the tracheobronchial tree and lower locoregional recurrences when compared to transhiatal esophagectomy. Disadvantages include the severe morbidity and mortality associated with anastomotic leak into the thorax or mediastinum, thoracic duct leak, an overall higher incidence of pulmonary complications, and the occasional necessity to expand to a cervical exposure if a proximal margin cannot be cleared [42]. This final addition constitutes a so-called “three-field” esophagectomy (i.e., McKeown), effectively combining the advantages of both approaches. Perhaps counterintuitively, however, the safety profile of this third resection technique is actually inferior to either prior method, likely due to its application in only the most challenging tumors from a surgical standpoint. Again, as in all types of esophagectomy, a feeding jejunostomy is placed in all patients.
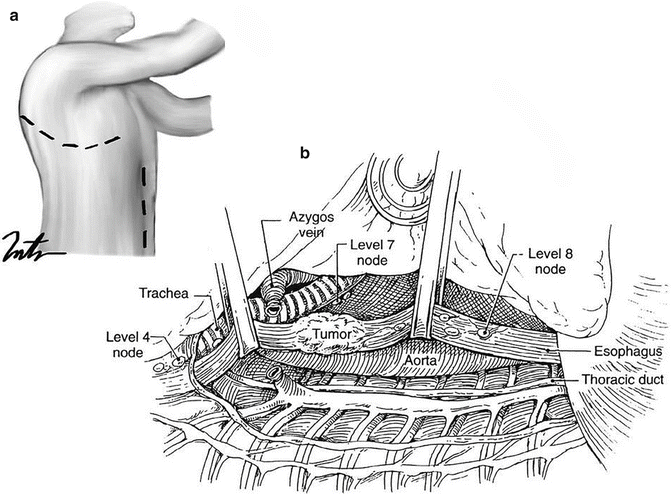

Fig. 8.8
(a) An Ivor-Lewis approach includes an abdominal and a right thoracic incision (Courtesy of Thuy-Tien Chu). (b) The azygos vein is ligated, the thoracic esophagus is mobilized, and the mediastinal lymph nodes are retrieved (Reprinted from Ref. [40]. With permission from Elsevier)
On occasion, a left thoracotomy instead of a right thoracotomy is performed for GEJ and cancer involving the cardia of the stomach. In such a situation, esophagogastric anastomosis is performed just superior to the inferior pulmonary veins. The disadvantage with this approach is the presence of the aortic arch, which limits the surgeon’s ability to perform a higher anastomosis, although some have advocated tunneling the conduit behind the aortic arch. The left thoracotomy approach is not as widely performed as the other approaches, and it is mentioned here only for completeness.
Minimally Invasive Techniques
As with the adaptation of many oncologic resections from traditional open approaches to minimally invasive methods, esophagectomy has been well documented in the literature as a feasible and oncologically sound undertaking [43, 44]. Preserving the steps and general architecture of the original operation, minimally invasive esophagectomy (MIE) can be performed with or without thoracoscopic dissection of the intrathoracic esophagus. Though no prospective randomized trials to compare MIE and open techniques exist, several series have indicated that MIE is safe and effective, allowing an oncologically sound resection (evidenced by extent of lymphadenectomy and margin clearance) with a comparably safe risk profile when compared to historical standards [45]. Similar to the advantages afforded by minimally invasive techniques employed in other operations, decreased postoperative pain, faster return of bowel function, and improved cosmesis have been achieved in comparison to the traditional open methods [45]. This is especially true in the avoidance of thoracotomy provided by thoracoscopic resection and reconstruction.
Extent of Lymphadenectomy
Completeness of lymphadenectomy has been long considered the obligate partner to the successful resection of primary tumors, and this concept has been the subject of some scrutiny over the preceding years. With the refinement of neoadjuvant therapies, there has been a general sense of devaluation placed upon radical lymphadenectomies so prevalent in historical view. Driving this scrutiny is the inherent difference between the operative approaches in regard to emphasis placed on accessibility to lymph nodes; several studies have indicated that the transthoracic approach allows a more thorough lymphadenectomy, and indeed, the number of lymph nodes retrieved is often higher compared to the transhiatal approach. Surgeons who endorse the transhiatal approach despite the reproducibly lower number of lymph nodes harvested submit the supporting literature that long-term outcome is not influenced by the number of nodes removed at surgery [46, 47].
Alternate Methods of Reconstruction
Occasionally, either due to tumor- or anatomic-related considerations, the stomach is not a suitable candidate for esophageal reconstruction. In these instances, the most commonly employed conduit is an interposition of a segment of the colon either in the posterior mediastinum or the anterior mediastinum [48, 49] (Figs. 8.6 and 8.9). Similarly, an isolated jejunal segment can also be used to provide the necessary hollow viscus to reconstitute the upper digestive tract. In the case of the former, special preparation in the preoperative phase is required to verify the integrity of the colon for eventual use. Colonoscopy to ensure the absence of neoplasms and mesenteric angiography to evaluate the vascular inflow of the future pedicle are necessary steps if such a reconstruction is planned. As for the former, several techniques of improving the vascular supply of what is often a free graft have been described. These often involve augmenting arterial inflow to the intestinal mesentery by direct arterial anastomosis to the common carotid artery. Though quality of life is somewhat limited by these alternate reconstruction techniques, they do offer a preferable alternative to lifelong intestinal discontinuity and the presence of a cervical esophagostomy or “spit fistula.”
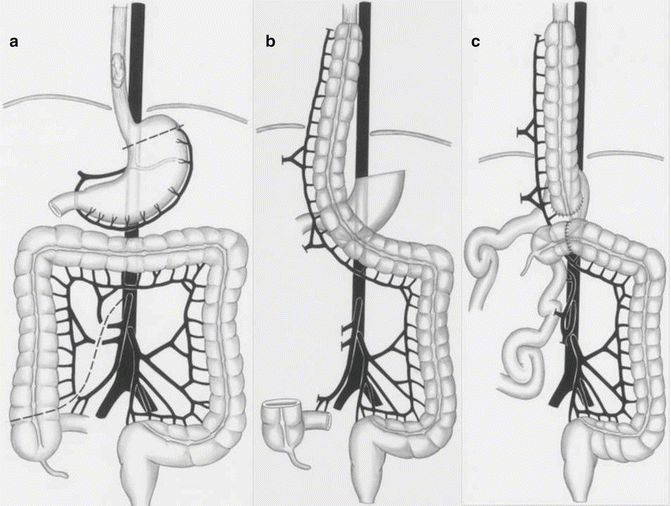

Fig. 8.9
Technique of using the right colon as an alternative conduit (Reprinted from Ref. [49]. With permission from Wolters Kluwer Health)
Radiation
The rationale for radiation therapy is for the definitive management for unresectable cases or to sterilize microscopic disease near the primary tumor and in regional lymph nodes as part of a neoadjuvant regimen. The use of chemoradiation for nonsurgical candidates was established by the Radiation Therapy Oncology Group (RTOG) 8501 trial where radiation alone was compared to concurrent chemoradiation [50]. The trial showed a dismal 5-year overall survival rate of 0 % versus 27 % for the concurrent arm.
Given the relatively low definitive radiation dose, several trials have investigated the role of radiation dose escalation to improve outcomes. The intergroup 0213 trial randomized patients to receive 64.8 Gy versus 50.4 Gy [51]. The results of this trial found no significant difference between the two arms in median survival (13.0 vs. 18.1 months), 2-year overall survival (31 % vs. 40 %), or locoregional failure (56 % vs. 52 %). This trial has been criticized as 7 of the 11 treatment-related deaths occurred prior to receiving 50.4 Gy.
RTOG 9207 was a phase I/II study of concurrent chemotherapy followed by a brachytherapy boost [52]. Brachytherapy is a radiation technique that uses a point source of radioactive material that is brought close to the tumor. These sources have a limited range and are often used to deliver high conformal doses while sparing normal tissues. Unfortunately, a 12 % incidence of fistula formation was found from this technique and thus has not been readily adopted. Although efforts to dose escalate radiation have failed, it is important to note that approximately a quarter of unresectable patients can be cured with definitive chemoradiation.
For surgical candidates, there is some controversy regarding the benefit of neoadjuvant chemoradiation over surgery alone as several clinical trials failed to demonstrate an advantage with the neoadjuvant approach (Univ. of Michigan [53], European Organisation for Research and Treatment of Cancer (EORTC) [54], Univ. of Ulsan in Korea [55], and the combined Trans Tasman Radiation Oncology Group (TROG) and Australasian Gastro-Intestinal Trials Group (AGITG [56])). In contradistinction, an older study by Walsh et al. at St. James Hospital (Ireland) found an advantage with chemoradiation [57], and several recent studies including the Cancer and Leukemia Group B (CALGB) 9781 [58], the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial [59], as well as a meta-analysis [60] found a significant survival advantage to trimodality therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree