Outline
Incidence, Epidemiology, and Etiology
Signs, Symptoms, and Attempts at Early Detection (Screening)
Diagnostic Techniques and Staging
Therapeutic Options for Primary Treatment
Borderline Malignant Epithelial Neoplasms
Treatment of Malignant Epithelial Neoplasms
Extraovarian Peritoneal Serous Papillary Carcinoma
Follow-up Techniques and Treatment of Recurrences
Use of CA-125 Levels and Other Tumor Markers
Chemotherapy for Recurrent Disease and Targeted Therapies
Key Points
- 1.
Ovarian cancer is the most lethal of all gynecologic cancers. Elderly women are more likely to develop ovarian cancer than young women.
- 2.
Oral contraceptives decrease the incidence of ovarian cancer substantially.
- 3.
Ovarian cancer may be inherited with increased risk being caused by a mutation in BRCA1 or BRCA2 , mismatch repair defect, or any number of other less common genes tested in the current panels.
- 4.
A combination of surgery and chemotherapy is almost always recommended for treatment. The order depends on the extent of disease, volume, and location.
- 5.
Significant strides have been made in survival as treatment and surgery have evolved.
Malignant neoplasms of the ovary are the cause of more deaths than any other female genital tract cancer. In the United States, there were approximately 21,290 new cases and 14,180 deaths in 2015 as a result of ovarian cancer. Ovarian cancer accounts for 5% of all cancers among women. In the United States, deaths from this cause occur at a rate of one every 44 minutes, and this disease will develop in one of every 68 women. Doctors and patients alike continue to be frustrated by our lack of understanding of the factors that lead to ovarian cancer and the failure to achieve a significant reduction in mortality.
Classification
The most practical classification scheme is based on the histogenesis of the normal ovary, shown in Table 11.1 . The early development of the ovary is divided into four stages. During the first stage, undifferentiated germ cells (primordial germ cells) become segregated and migrate to the genital ridges, which are bilateral thickenings of coelomic epithelium. The second stage consists of proliferation of the coelomic epithelium and the underlying mesenchyme. During the third stage, the ovary becomes divided into a peripheral cortex and a central medulla. The fourth stage is characterized by the development of the cortex and involution of the medulla. The histogenetic classification categorizes ovarian neoplasms with regard to their derivation from coelomic epithelium (epithelial ovarian cancer), germ cells (germ cell tumors), and mesenchyme (sex cord-stromal tumors).
Neoplasms derived from the coelomic epithelium
Neoplasms derived from germ cells
Neoplasms derived from specialized gonadal stroma
Neoplasms derived from nonspecific mesenchyme
Neoplasms metastatic to the ovary
|
The majority (85%–90%) of malignant ovarian tumors are epithelial. They can be grouped into predominant histologic types as follows:
| Serous cystadenocarcinoma | 42% |
| Mucinous cystadenocarcinoma | 12% |
| Endometrioid carcinoma | 15% |
| Undifferentiated carcinoma | 17% |
| Clear cell carcinoma | 6% |
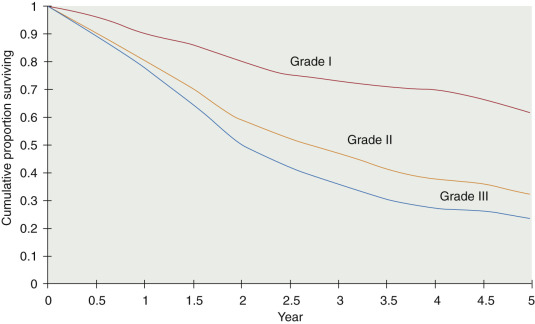
The specific malignant histologic type has less prognostic significance than clinical stage, extent of residual disease, and histologic grade. Histologic grade, however, is an important independent prognostic factor in patients with epithelial tumors of the ovary ( Fig. 11.1 ). Because survival for patients with grade II and III tumors is more closely related, research studies frequently stratify by low-grade (grade I) and high-grade (grade II and III) tumors.
Incidence, Epidemiology, and Etiology
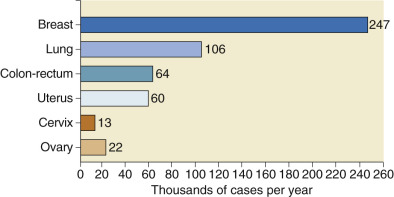
Approximately 22% of gynecologic cancers are of ovarian origin ( Fig. 11.2 ), but 47% of all gynecologic cancer deaths occur in women who have ovarian cancer. Cancer of the ovaries is the fifth most frequently occurring fatal cancer in women in the United States and also ranks high as a cause of cancer death in Canada, New Zealand, Israel, and countries of northern Europe. Ovarian cancer will develop in approximately 14 of every 1000 women in the United States older than age 40 years ( Table 11.2 ), but only four of the 14 will be cured. The remaining patients often develop repeated bouts of intestinal obstruction as the tumor spreads over the surface of the bowel, develop inanition and malnutrition, and quite literally starve. In a review of mortality trends in the United States, age-adjusted (to the 2000 US standard population) ovarian cancer mortality rates from 1975 to 2002 showed the absolute number of deaths increased, consistent with a growing and aging population. Over the past 30 years, mortality rates decreased for women younger than 65 years but increased for women older than 65 years, with some plateauing over the past 10 years. These changes may result from increased use of oral contraceptives in younger patients and a shifting of the survival curve to the right. Even when matched for stage, survival was worse in older women. In some cases, this may be a result of less aggressive treatment in the older woman and the higher percentage of low-grade disease in younger patients. Age-adjusted death rates were higher in whites than in blacks. Asians and Pacific Islanders, American Indian and Alaskan natives, and Hispanics have lower death rates than blacks.
| Site | Probability |
|---|---|
| All sites | 1 in 3 |
| Breast | 1 in 9 |
| Cervix | 1 in 154 |
| Uterine corpus | 1 in 41 |
| Lung | 1 in 18 |
| Ovary | 1 in 81 |
| Colon–rectum | 1 in 26 |
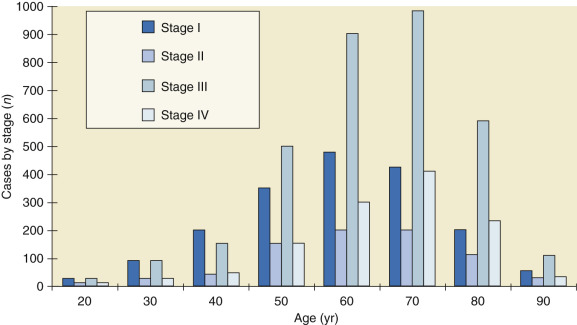
Malignant neoplasms of the ovaries occur at all ages, including infancy and childhood ( Fig. 11.3 ). Throughout childhood and adolescence, the rate of death from ovarian carcinoma in the United States is exceeded by those for leukemia; lymphomas; and neoplasms of the central nervous system, kidney, connective tissue, and bone. The major histologic types occur in distinctive age ranges ( Table 11.3 ). Whereas malignant germ cell tumors are most commonly seen in girls younger than age 20 years, epithelial cancers of the ovary are primarily seen in women older than age 50 years. Beginning at age 45 to 49 years, with a rate of 16.4 cases per 100,000, incidence increases progressively with age. The rate increases after the age of 60 years to 40 cases per 100,000 and then peaks at 61 cases per 100,000 in the age group 80 to 84 years. The largest absolute number of patients with ovarian cancer is found in the age group 60 to 64 years; more than one-third of the cases occur in patients 65 years or older. Elderly women are more likely than younger women to be in advanced stages of ovarian cancer at initial diagnosis, and the 5-year relative survival rate for elderly women is about half the rate (28.4%) observed in women younger than 65 years (56.6%).
| Type | Age <20 Yr (%) | Age 20–50 Yr (%) | Age >50 Yr (%) |
|---|---|---|---|
| Coelomic epithelium | 29 | 71 | 81 |
| Germ cell | 59 | 14 | 6 |
| Specialized gonadal stroma | 8 | 5 | 4 |
| Nonspecific mesenchyme | 4 | 10 | 9 |
Epidemiologic evidence strongly suggests that environmental factors play a major role in developing epithelial cancer of the human ovary. Highly industrial countries have the highest reported incidence, and physical or chemical products of industry may be causative factors. A notable exception is highly industrialized Japan, where rates for ovarian cancer have been among the lowest recorded in the world. Of interest, the incidence of ovarian cancer increases in Japanese immigrants to the United States and their offspring, eventually approaching that of Anglo-Saxon whites by the second and third generations. This suggests that causative carcinogens may be present in the immediate environment, such as food, personal customs, and other influences that change gradually during the cultural transition. To date, there are no firm clues as to which dietary items or other environmental contacts are specifically carcinogenic for the ovary.
A decreased incidence of ovarian cancer is related to less frequent ovulation. Danforth and coworkers prospectively examined risk of ovarian cancer in the Nurses’ Health Studies and found that women who never breastfed faced a 1.5-fold risk of ovarian cancer compared with women who breastfed for longer than 18 months. These observations coincided with data illustrating a reduction in the disease incidence with the use of oral contraceptives ( Table 11.4 ). The hypothesis that oral contraceptive use reduced the risk of epithelial ovarian cancer was suggested by Casagrande and associates in 1979. In 1982, Rosenberg and colleagues reported a case-control study in which combination oral contraceptives were used by 26% of the patients with ovarian cancer and 35% of the control participants. The relative risk estimate for combination oral contraceptive use was 0.6 ( Table 11.5 ). The reduction in risk appeared to persist for as long as 10 years after use has ceased and to be greater for longer durations of use, but these results were not statistically significant. Their conclusion was that the use of combination oral contraceptives protects against epithelial ovarian cancer. The theory of “incessant ovulation” suggests that the epithelial lining of the ovary may be sensitive to the events of ovulation, which in turn can act as a promoting factor in the carcinogenic process. Gross and Schlesselman determined the effect of oral contraceptive use on the cumulative incidence of epithelial ovarian cancer from ages 20 to 40 years, 20 to 50 years, and 20 to 55 years and stratified these age categories by family history and parity. The number of epithelial ovarian cancer cases estimated to occur per 100,000 oral contraceptive users decreased with increasing duration of use. Their results suggest that 5 years of use by nulliparous women can reduce their ovarian cancer risk to the level seen in parous women who never use oral contraceptives, and 10 years of use by women with a positive family history can reduce their risk to a level below that of women whose family history is negative and who never use oral contraceptives. On the basis of these data, the recommendation is strongly made that premenopausal patients with risk factors for ovarian cancer use oral contraceptives when pregnancy is not being sought.
| Term Pregnancies ( n ) | Cases | % | Population Studies | Odds Ratio | |
|---|---|---|---|---|---|
| Controls | Percentage | ||||
| 0 | 322 | 24 | 765 | 14 | 1.44 |
| 1 | 164 | 12 | 605 | 11 | 1.6 |
| 2 | 376 | 28 | 1515 | 27 | 1.53 |
| 3 | 265 | 19 | 1259 | 22 | 1.48 |
| 4 | 135 | 10 | 774 | 14 | 1.36 |
| 5 | 56 | 4 | 345 | 6 | 1.33 |
| 6 | 45 | 3 | 346 | 6 | 1.29 |
| Duration of Oral Contraceptive Use | Women Who Developed Ovarian Cancer ( n ) | Controls ( n ) | Relative Risk |
|---|---|---|---|
| Never | 242 | 1532 | 1.4 |
| 3–6 months | 26 | 1280 | 0.6 |
| 7–11 months | 14 | 1134 | 0.7 |
| 1–2 years | 65 | 1602 | 0.7 |
| 3–4 years | 40 | 1397 | 0.6 |
| 5–9 years | 39 | 1594 | 0.4 |
| ≥10 years | 13 | 1328 | 0.2 |
Over many years, studies have disagreed as to the association between fertility drug exposure and ovarian cancer. Whittemore and associates noted that infertile women treated with infertility drugs had a 2.8 times increased risk of ovarian cancer compared with women without infertility. The risk was higher among women who did not become pregnant compared with the parous women. Specific drug exposure was not documented. Subsequent studies by Franceschi, Rossing, Venn, Shishan and colleagues disagreed as to whether induction with clomiphene or gonadotropins were associated with increased risk. The largest reported cohort drew 126 women with invasive epithelial ovarian cancer from a cohort of 54,362 women treated at all Danish infertility clinics from 1963 to 1968. The effects of four separate groups of fertility drugs were evaluated individually and in combination (gonadotropins, clomiphene citrate, human chorionic gonadotropin, and gonadotropin-releasing hormone). No convincing associations were found between use of these fertility drugs and ovarian cancer risk. The published data suggest that there is probably little relationship between fertility drug use and ovarian cancer. Further investigations are underway to clarify this association. The concept is appealing when one considers the reduction in incidence noted with the use of oral contraceptives and parity.
Whittemore and associates, in a population-based study, noted protection against ovarian cancer with increasing parity (see Table 11.4 ). McGowan and colleagues, on the basis of a study of 197 women with ovarian cancer, estimated that nulligravidae were 2.5 times more likely to have malignant ovarian tumors and 2.9 times more likely to have ovarian carcinoma of low potential malignancy than were women who had been pregnant three or more times. The risk of ovarian cancer in their series was further reduced among women who had been pregnant at least once. One possible interpretation could be that the endocrine state of pregnancy protects against ovarian cancer and that the lack of this protection places infertile women at higher risk for ovarian cancer. A second explanation could be that infertility and ovarian cancer result from a similar genetically abnormal gonadal status. This theory would explain why infertile women are more at risk than never-married and never-pregnant women.
In the large prospective Cancer Prevention Study II, Rodriguez and coworkers evaluated the use of acetaminophen and a possible relationship to ovarian cancer mortality. Women who reported using acetaminophen daily had a death rate from ovarian cancer 45% lower than that of women reporting no use (relative risk = 0.55; confidence interval [CI] = 0.27–1.09). In a case-control study, Cramer and associates had previously reported that the odds ratio was 0.52 for development of ovarian cancer among women who used the drug at least once a week for at least 6 months; lowest risk was found in women who used the drug daily and for more than 10 years. Cramer et al. also noted that the odds ratio of ovarian cancer was 0.75 (CI 0.52–1.10) for aspirin and 1.03 for ibuprofen.
Parazzini and colleagues studied the influence of various menstrual factors on the risk of epithelial ovarian cancer. They reported that the risk rose with later age at menopause and with early menarche. Studies of dietary fat have been inconclusive, and other dietary factors are not well characterized. Although the Women’s Health Initiative investigators reported a low-fat diet was associated with up to a 40% reduction in ovarian cancer risk, several large cohort studies have not been able to demonstrate this. Because diet and exercise are potentially modifiable risk factors, continued investigation into their role is warranted.
Some have suggested that ovarian cancer may be initiated by a chemical carcinogen through the vagina, uterus, and fallopian tubes and that damaged ovarian surface epithelium from ovulation induces inflammation and mutation susceptibility. For years, Woodruff and coworkers suggested this hypothesis of migration of chemical carcinogens from the vagina to the pelvic peritoneum. Venter demonstrated with the use of radionuclides that upward migration is possible. Certainly, many different chemical substances could migrate to vulvovaginal areas and be implicated in carcinogenesis. The agent most extensively studied has been talc powder, but many of the studies that noted a slight increased risk of ovarian carcinoma in association with talc did not adjust for other factors (oral contraceptive use, family history) that are related to ovarian cancer. Others included low malignant potential tumors with their invasive cancers or did not correct for integrity of the female genital tract status. The relationship (if any) of talc to ovarian cancer would appear to be minimal at best.
No epidemiologic or experimental evidence exists to incriminate viruses in the development of neoplasms of the human ovary. Attempts to isolate viruses from cultures of human ovarian cancer cells have been unsuccessful to date. Because of its gonadotropic properties, mumps virus is an obvious candidate among known viruses for oncogenic activity in the ovary. At present, however, the evidence for mumps virus as an etiologic agent in ovarian cancer remains speculative.
Familial Ovarian Cancer
Familial hereditary ovarian cancer currently falls into three categories:
- 1.
Site-specific familial ovarian cancer
- 2.
Hereditary breast-ovarian cancer syndrome, in which there is an increased incidence of breast and ovarian carcinomas alone or in combination
- 3.
Lynch syndrome type II, in which family members may develop a variety of cancers, including colorectal, endometrial, and ovarian cancer
Inherited genetic mutations are associated with approximately 10% of women who develop ovarian cancer. The mutation is inherited in an autosomal-dominant fashion (maternal or paternal transmission), and multiple family members are affected over several generations. First-degree relatives (mother or sister) are frequently involved.
True hereditary ovarian cancer and breast cancer mainly result from mutations of BRCA1 and BRCA2 genes. Individuals with these mutations ( BRCA1 and BRCA2 ) generally have a germline mutation (inherited mutated copy of the gene) in contrast to the more common somatic mutation (noninherited, or acquired) in most patients with ovarian cancer. BRCA1 mutations are located on the long arm of chromosome 17q, and BRCA2 mutations are located on chromosome 13q12. Lynch II syndrome arises as a result of inherited mutation in a family of DNA repair genes (MSH2, MLH1, PMS1, PMS2); this group accounts for only a small number of inherited ovarian cancers. Initial evaluation of these germline mutations suggested an estimated risk of ovarian cancer of 39% to 46% for carriers of the BRCA1 mutation but a lower rate of 10% to 20% for carriers of the BRCA2 mutation. With more than 400 mutations identified in the BRCA1 and BRCA2 genes along with multiple polymorphisms, estimating risk is difficult. In most women with a strong family history who do develop ovarian cancer, the disease is sporadic in nature and not inherited. Houlston and colleagues evaluated 391 women who were self-referred to a screening clinic for familial ovarian cancer. Of these women, 290 (74%) appeared to have no clear inheritance pattern, and there was no increased risk of cancer in the first-degree relatives. There were 82 (21%) pedigrees compatible with multisite cancer family syndrome with a relative risk of about 6, or development of ovarian cancer in 1 of 16. In the 19 families with site-specific ovarian cancer, the relative risk for first-degree relatives was 39, with a lifetime risk of 1 in 2. Kerlikowske and associates reviewed published studies of familial ovarian cancers and used ovarian cancer incidence data from the Surveillance, Epidemiology, and End Results (SEER) database to estimate lifetime probabilities of ovarian cancer in these families. They noted that the lifetime probability of ovarian cancer for a 35-year-old woman was 1.6% in an individual without a family history of ovarian cancer. It increased to 5% if she had one relative and 7% if she had two relatives with ovarian cancer. These and other studies by Bourne and colleagues were based on family history pedigree and preceded BRCA1 and BRCA2 testing.
Claus and associates, using a previously fit autosomal-dominant genetic model, evaluated the Cancer and Steroid Hormone Study of 4730 patients with breast cancer aged 20 to 54 years and 4688 control subjects to estimate the proportions of breast and ovarian cases in the general population that are likely to result from breast and ovarian cancer susceptibility genes. About 10% of patients with ovarian cancer in the general population were estimated to be carriers of a breast or ovarian cancer susceptibility gene. The risks for ovarian cancer in carriers to the age of 60 years and 80 years were estimated to be about 10% and 27%, respectively. Carriers were predicted to have at least a 15-fold age-specific risk of ovarian cancer compared with noncarriers. Some ethnic groups appear to be at high risk for gene mutation, and the Ashkenazi Jews have been most extensively studied. In a large study by Struewing and associates, 5318 Jewish subjects had BRCA1 and BRCA2 evaluated, identifying 120 carriers. Carriers had a significantly elevated risk, with an estimated risk of 2% by age 50 years and 16% by age 70 years compared with the noncarrier risk of 0.4% by 50 years and 1.6% by 70 years. Metcalfe and colleagues performed BRCA testing for three founder mutations in 2080 self-identified Jewish women and found the BRCA mutation rate to be 1% (22 women) in an unselected population.
Frank and associates evaluated 283 women with breast cancer before age 50 years or ovarian cancer at any age and at least one first- or second-degree relative with either diagnosis for BRCA1 followed by BRCA2 analysis. Mutations were identified in 94 women (39%), including 59 of 117 (50%) from families with ovarian cancer and 35 of 121 (29%) from families without ovarian cancer. In women with breast cancer, mutations in BRCA1 and BRCA2 were associated with a 10-fold increased risk of subsequent ovarian cancer. Mutations were noted in 24 of 38 women (63%) who had ovarian cancer. In women from breast–ovarian cancer families, 30 of the germline mutations occurred in BRCA2 . Of note in this study, the authors did not find Ashkenazi ancestry significantly increased in the identification of BRCA1 or BRCA2 mutation, suggesting the importance of a strong family history.
In considering women at high risk, two questions should be addressed: (1) Who should be tested? and (2) Are there screening or prophylactic measures that can be applied to decrease the risk? A family history of breast or ovarian cancer, particularly before the age of 50 years in a first-order relative, and Ashkenazi Jewish ancestry, are risk factors for BRCA1 or BRCA2 mutations. Family history, both maternal and paternal, should be obtained (three generations is desired), and age at diagnosis should be noted. Pathology reports or death certificates verify accuracy of history; there can be considerable errors from history alone. Lifetime empirical risks can be determined from this information and may suggest that testing for a gene mutation is indicated. The extensive evaluation of family history and counseling should be done before genetic testing. Interpretation and plan of action that will influence medical management should be discussed in detail before genetic testing is performed. Testing of family members known to have cancer before testing of the cancer-free members is recommended. If a suspect mutation is found in the family member with cancer, the relatives without cancer can be tested for that specific mutation. If the family member with cancer does not have a mutation, other relatives probably will not benefit from testing. In one study, women sought DNA testing most commonly because of concern about the potential cancer risk to their children and secondly for their own cancer surveillance and prevention.
In a consensus statement of the Cancer Genetics Studies Consortium, annual or semiannual screening with transvaginal ultrasonography (TVS) and determination of serum carcinoma antigen 125 (CA-125) levels beginning at age 25 to 35 years are recommended for BRCA1 mutation carriers. Both of these tests carry relatively high false-positive and false-negative rates with low positive predictive value. Narod and associates found that oral contraceptives protected against ovarian cancer in patients with mutation in either the BRCA1 or BRCA2 gene.
Risk-reducing salpingo-oophorectomy after completion of childbearing, or age 40 years, in women at high genetic risk is associated with a significant reduction in the risk of BRCA1- or BRCA2 -associated ovarian cancer. A National Institutes of Health (NIH) Consensus Conference recommended that women with two or more first-degree relatives with ovarian carcinoma be offered prophylactic oophorectomy after completion of childbearing or age 35 years. Prophylactic oophorectomy does not prevent subsequent primary peritoneal cancer, in which failure rates of 4% to 5% at 20 years after risk-reducing salpingo-oophorectomy (RRSO) were reported by Finch and colleagues. Rebbeck and associates clarified risk reduction estimates in their 2009 meta-analysis. They found RRSO was associated with an 80% risk reduction in BRCA1- or BRCA2 -associated ovarian, fallopian tube and peritoneal cancer, and a 50% risk reduction in BRCA1- or BRCA2 -associated breast cancer.
A 2005 study by Pal and colleagues suggested that previous studies may have underestimated the frequency of BRCA1 and BRCA2 mutations. Of 209 patients with invasive cancer, 32 patients (15%) had mutations of BRCA1 ( n = 20) or BRCA2 ( n = 12). Based on their data, they suggested it may be reasonable to offer genetic counseling to any woman with an invasive, nonmucinous epithelial ovarian cancer, and subsequent studies have supported this observation. A 2014 Society of Gynecologic Oncologists (SGO) Clinical Practice Statement on Genetic Testing for Ovarian Cancer recommended women diagnosed with epithelial ovarian, tubal, and peritoneal cancers receive genetic counseling and be offered genetic testing even in the absence of a family history.
The Gynecologic Oncology Group (GOG) has conducted a study (GOG 199) of 2605 women at increased risk for developing ovarian cancer, either by known BRCA mutation or family history risk factors. Patients were enrolled after choosing either prophylactic oophorectomy or screening, and both groups followed with CA-125 levels and Risk of Ovarian Cancer Algorithm (ROCA) assessment. The study was designed to evaluate both the prospective incidence of new and occult ovarian cancers and to assess the ROCA and quality of life. In 2014, Sherman and colleagues reported a portion of the analysis of high risk women older than 30 years of age who elected RRSO. Clinically occult cancers were detected in 2.6% of high risk women ( BRCA1 mutation carriers, 4.6%; BRCA2 mutations carriers, 3.5%; noncarriers, 0.5%), and further results from this trial will be forthcoming.
Signs, Symptoms, and Attempts at Early Detection (Screening)
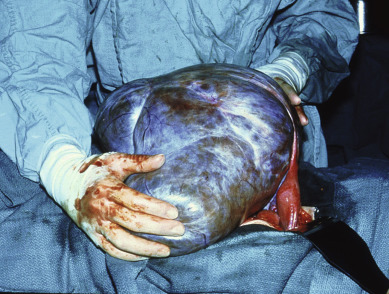
Although the wide range of ovarian tumor types generally presents in a similar manner, the diagnosis of early ovarian cancer is often more a matter of chance than a triumph of the scientific method. As the ovary enlarges ( Fig. 11.4 ), there may be progressive compression of the surrounding pelvic structures, producing vague abdominal discomfort, urinary frequency, and “pelvic pressure” ( Table 11.6 ). The insidious onset of ovarian cancer needs no elaboration. As the neoplasm reaches a diameter of 10 to 15 cm, it may account for abdominal enlargement. The notions that the development of ovarian cancer is “silent” and there are no early symptoms of ovarian cancer are a focus of the patient advocacy “listen for the whisper” educational efforts. Vague symptoms may be present for several months before the diagnosis. Such complaints are usually not recognized as anything more than gas or indigestion. A high index of suspicion is warranted in all women between the ages of 40 and 69 years who have persistent gastrointestinal (GI) symptoms that cannot be diagnosed. The majority of such nonspecific complaints are nonmalignant, causing the primary care physician to dismiss the possibility of ovarian cancer in many cases. Too frequently, it is only when the patient has gross abdominal enlargement marking the occurrence of ascites and abdominal carcinomatosis that appropriate diagnostic evaluation is undertaken.
| Symptom | Relative Frequency |
|---|---|
| Abdominal swelling | 60% |
| Abdominal pain | 40% |
| Dyspepsia | 30% |
| Urinary frequency | 30% |
| Weight change | 10% |
Methods for early diagnosis have been investigated in limited studies using cul-de-sac aspiration for peritoneal cytologic assessment and frequent pelvic examinations. All of these endeavors have failed to show a significant impact on early diagnosis of this disease. Most ovarian neoplasms grow quickly and painlessly. A persistent ovarian enlargement should be an indication to consider surgery, and the diagnosis is made by the pathologist. The size of the tumor does not indicate the severity of disease; indeed, many of the largest neoplasms are histologically benign, most commonly mucinous cystadenoma. Moreover, many large adnexal masses may be of nonovarian etiology. Nonovarian causes of apparent adnexal masses are diverticulitis, tubo-ovarian abscess, carcinoma of the cecum or sigmoid, pelvic kidney, and uterine or intraligamentous myomas. At the time of surgery, it may be difficult to discern the malignant potential of a particular ovarian neoplasm ( Table 11.7 ). There is sufficient overlap of morphologic criteria to cause confusion. The diagnosis rests with the histologic examination of the specimen, and the error rate of frozen section ranges from 5% to 15%.
| Benign | Malignant | |
|---|---|---|
| Surface papilla | Rare | Very common |
| Intracystic papilla | Uncommon | Very common |
| Solid areas | Rare | Very common |
| Bilaterality | Rare | Common |
| Adhesions | Uncommon | Common |
| Ascites (100 mL) | Rare | Common |
| Necrosis | Rare | Common |
| Peritoneal implants | Rare | Common |
| Capsule intact | Common | Infrequent |
| Totally cystic | Common | Rare |
Immunologic diagnosis of subclinical ovarian cancer by means of identification of specific tumor-associated antigens in the serum has yet to materialize. Several tumor-associated antigens, including CA-125, have been identified and purified. Research identifying patterns of protein fragments (proteomics) in patients with ovarian carcinoma requires validation in larger trials and a cost-effective way to implement.
It has been suggested that every woman should have a periodic pelvic examination, pelvic ultrasound examination, and CA-125 test to make sure she does not harbor an occult ovarian cancer. Enthusiasm for early detection of ovarian cancer is laudable. However, it has been calculated that 10,000 routine pelvic examinations would be required to detect one early ovarian cancer in a population of asymptomatic patients. For a low-incidence cancer such as ovarian cancer, cost-effective screening strategies require a positive predictive value (PPV) greater than 10%. This generally requires a technique specificity of greater than 99%, with a high sensitivity of greater than 75%. The majority of early trials of screening techniques did not meet this metric. Jacobs and colleagues studied 22,000 postmenopausal women screened with CA-125 determinations and have reported follow-up observed for a mean of 6.76 years; 767 women (3.5%) had elevated CA-125 values, of whom 49 (6%, or 0.0022% of the total screened) had cancer. One-third of these were early stage. Overall specificity was 97%, and sensitivity at 1 year and 7 years of follow-up was 75% and 57%, respectively. The PPV of 3% was not high enough to be an effective screening method, and there is no current role for screening with CA-125 levels in unselected women. Many conditions of a benign nature, and most GI tract malignant neoplasms may elevate the CA-125 concentration ( Table 11.8 ). The value of ultrasonography in screening for early ovarian carcinoma has received much attention. Campbell and coworkers reported early detection of five primary ovarian cancers in approximately 5000 women screened by abdominal ultrasonography ( Table 11.9 ). DePriest and associates studied postmenopausal women or women with a family history of ovarian cancer (24% of women) with TVS. They screened 6470 women with 14,829 scans; 90 women had persistent abnormalities on TVS and underwent surgery. Six primary ovarian cancers were found (five stage I and one stage IIIb), and only four were epithelial. Sensitivity was 86%, and specificity was 99%, with a PPV of only 7% but with a negative predictive value (NPV) of 99.9%.
| Gynecologic | Nongynecologic |
|---|---|
| Acute pelvic inflammatory disease | Active hepatitis |
| Adenomyosis | Acute pancreatitis |
| Benign ovarian neoplasm | Chronic liver disease |
| Endometriosis | Cirrhosis |
| Functional ovarian cyst | Colitis |
| Meigs’ syndrome | Congestive heart failure |
| Menstruation | Diabetes (poorly controlled) |
| Ovarian hyperstimulation | Diverticulitis |
| Unexplained infertility | Mesothelioma |
| Uterine myoma | Nonmalignant ascites |
| Pericarditis | |
| Pneumonia | |
| Polyarteritis nodosa | |
| Postoperative period | |
| Renal disease | |
| Rodent exposure (HAMA response) | |
| Systemic lupus erythematosus |
| Screened | Prevalence of Detected Primary Cancer | Screen-Detected Cancers per 100,000 | Stage I | |
|---|---|---|---|---|
| GENERAL POPULATION | ||||
| Ultrasonography | 15,834 | 8 | 51 | 6 |
| Exclude LMP | 6 | 38 | 4 | |
| Multimodal * | 27,560 | 14 | 51 | 7 |
| Exclude LMP | 13 | 47 | 6 | |
| HIGH-RISK POPULATION | ||||
| Ultrasonography † | 4551 | 21 | 461 | 12 |
| With family history | 3146 | 15 | 477 | 9 |
| Exclude LMP | 8 | 254 | 2 | |
One strategy to improve the effectiveness of ovarian cancer screening would be to target populations at increased risk for the development of the disease, such as individuals with a positive family history of ovarian cancer. Bourne and colleagues reported the results of such a strategy in screening patients with TVS in combination with color-flow Doppler imaging and morphologic assessment. In the screening of 1601 patients, 57% required repeated TVS to confirm the presence of a mass. Six ovarian cancers were diagnosed (two stage I, three low malignant potential tumors, and one stage III). Karlan and associates reported screening of 597 patients with a family history of cancer by CA-125 measurement, TVS, and color-flow Doppler imaging. Initially, 115 patients had an abnormal finding on TVS, and 68 had abnormal CA-125 levels. After repeated TVS, because of abnormal findings of color-flow Doppler imaging, 19 patients underwent surgery. At the time of the report, one low malignant potential tumor had been diagnosed.
Bell and colleagues reviewed 25 studies on screening for ovarian cancer, 16 studies on women at average risk, and nine studies on women at higher risk. Many studies were limited by small numbers, imprecise methodology, scant follow-up, and single versus multimodality screening techniques. For women at average risk, 75% of primary cancers were stage I when they were detected with ultrasonography, and 50% were stage I when they were detected by multimodal screening (see Table 11.9 ). In women at higher risk, if low malignant potential tumors were excluded, only 25% were stage I. False-negative rates were also higher in the higher risk population than in the group at average risk. The false-negative data, when applied to a population with an annual incidence of 40 per 100,000, imply that 30 to 60 surgical procedures would be carried out for every cancer detected at annual gray-scale ultrasonography (assuming 100% sensitivity), and 2.5 to 15 surgical procedures would be carried out for every cancer detected by multimodal screening. Even if screening detects all ovarian cancers and these are treated with 100% success, the absolute reduction in mortality would be only 1 in about 2500 screened women per year. This is much smaller than the complication rate from unnecessary diagnostic surgery or recall for further tests.
Combining CA-125 levels with TVS has been explored as a means by which to improve predictive value. Skates and colleagues developed a risk of ovarian cancer (ROC) algorithm based on rising CA-125 that has been validated in a prospective cohort. Their initial validation PPV was high (19%); it has been applied to two large prospective trials. Buys and associates reported the ovarian portion of the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) trial, which randomized 78,216 women aged 55 to 74 years to annual screening with CA-125 and ultrasound versus usual care for up to 13 years. There was no reduction in the ovarian cancer mortality rate, and there was a 15% incidence of serious surgical complications in patients undergoing surgery for false-positive results. Retrospective application of the ROC algorithm by Pinsky and associates failed to show a survival benefit. Recently, Jacobs and colleagues reported results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), which randomized 202,638 women aged 50 to 74 years to annual multimodality screening (MMS) with CA-125 interpreted with the ROC algorithm, annual ultrasound screening, or no screening. Although the incidence of ovarian cancer and mortality was similar in the primary analysis, there was a significant mortality reduction with MMS in patients who lived longer than 7 years, likely because of detection of earlier stage cancers. These results require further follow-up, cost-effectiveness strategies, and validation in daily practice but give some further insight into early detection strategies.
Currently, however, there are no reliable data that screening for ovarian cancer is effective in improving length and quality of life in women with ovarian cancer. Another strategy to improve sensitivity and specificity in screening for ovarian cancer involves the use of multiple serum tumor markers. Because fewer than 50% of patients with stage I ovarian cancer will have an elevated CA-125 concentration and because CA-125 can be elevated by benign and malignant conditions, the addition of other markers in the screening strategy could potentially improve predictive value. Yurkovetsky and colleagues reported the results of their screening panel of four biomarkers, with 86% sensitivity at 98% specificity for detecting early-stage disease, and additional studies looking at additional biomarkers are ongoing. These techniques may also offer useful guidelines for predicting therapeutic response and for treatment selection. The NIH Consensus Development Conference on ovarian cancer screening in 1994 reached the following conclusions: There is no evidence available yet that the current screening modalities of CA-125 measurement and TVS can be used effectively for widespread screening to reduce mortality from ovarian cancer or that their use will result in decreased rather than increased morbidity and mortality. Routine screening has resulted in unnecessary surgery with associated risks. Clearly, it is important to identify and validate effective screening modalities. Currently available technology for screening should be used in the context of clinical trials to determine the efficacy of these modalities and their effect on ovarian cancer mortality. In addition, research must be continued to identify additional markers and imaging techniques that will be useful. Although these same recommendations still hold, we may have better techniques for screening because the data from ongoing trials mature. If a woman has one first-degree relative with ovarian cancer (making her lifetime risk of developing the disease 5%) but no clinical trials are available to her, she may think that despite the absence of prospective data, this is sufficient risk for her to be screened. This opportunity for screening should be available to the woman and her physician.
If a woman were undergoing pelvic surgery, removal of the ovaries at that time would almost fully eliminate her risk of ovarian cancer (although there remains a small risk of peritoneal cancer). If the woman is premenopausal, discussion of estrogen replacement therapy is important before removal of the ovaries because the risk of premature menopause and the potential for osteoporosis may outweigh the risk of ovarian conservation and the potential for ovarian cancer.
Diagnostic Techniques and Staging
Routine pelvic examinations detect only one ovarian cancer in 10,000 asymptomatic women. However, pelvic examination remains the most practical means of detecting early disease. Pain is usually a late complication; it is seen with early disease only in association with a complication such as torsion; rupture; or, rarely, infection. The physician should have a high index of suspicion for an early ovarian neoplasm in any ovary palpated in a menopausal patient. These patients should be considered for immediate laparoscopy or laparotomy when ultrasound examination findings suggest malignant change (eg, complex mass, >5 cm, or intracystic papillations).
To help with evaluating a patient with a complex pelvic mass, the American College of Obstetricians and Gynecologists (ACOG), in conjunction with the SGO, published guidelines in 2002 that could help direct referral to or consultation with a gynecologic oncologist. Physicians should perform a pelvic examination and imaging as appropriate for the patient’s symptoms or physical examination findings. For premenopausal women with a suspicious pelvic mass, referral to a gynecologic oncologist should be considered by at least one of the following: CA-125 level higher than 200 U/mL, ascites, abdominal or distant metastases, or one or more first-degree relatives with breast or ovarian cancer. For postmenopausal women with a concerning pelvic mass, consultation should be considered for any CA-125 elevation, ascites, nodularity or limited mobility, evidence of metastasis, or a first-degree relative with breast or ovarian cancer.
Routine laboratory tests are not of great value in the diagnosis of ovarian cancer but may help rule out other pelvic disorders. Pelvic ultrasonography may reveal calcifications consistent with myomas or benign teratomas. Colonoscopy and upper GI endoscopy may be helpful in patients who have lower or upper intestinal symptoms, respectively. Computed tomography (CT) with contrast enhancement may be helpful in identifying the extent of clinical disease. No imaging modality is conclusive, however, and suspicious pelvic masses, especially in postmenopausal women, should be evaluated surgically. In general, we recommend against needle biopsy of ovarian masses because such a procedure can result in spillage of malignant cells into the peritoneal cavity. Regardless of whether the fluid contains neoplastic cells, surgery will likely still be necessary to remove either the large benign neoplasm or to define the extent of the malignant process. In addition, up to 50% of ascitic fluid samples from patients with true ovarian malignant neoplasms will be negative for malignant cells on cell block analysis. Needle biopsy in a patient with a pelvic abdominal mass is therefore both unnecessary and potentially dangerous ( Fig. 11.5 ).
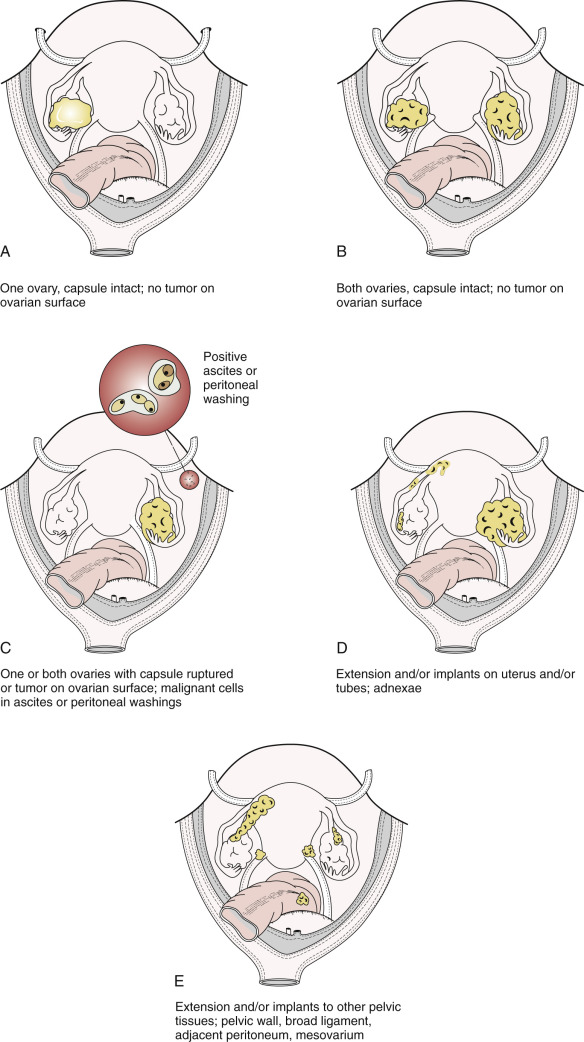
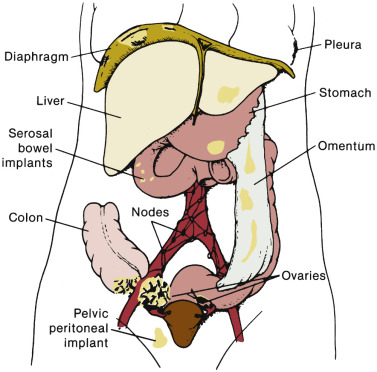
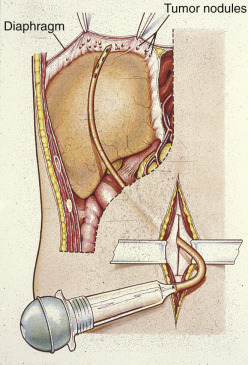
The staging of ovarian cancer is surgical ( Table 11.10 ) and based on the surgical and pathologic findings ( Fig. 11.6 ). A longitudinal midline incision is recommended to remove the neoplasm and to visualize the entire abdominal cavity, including the undersurface of the diaphragm. A Pfannenstiel incision is ill advised in a patient suspected of having an ovarian malignancy. Ovarian cancer classically involves the entire peritoneal cavity ( Fig. 11.7 ). Although lymphatic spread to retroperitoneal nodes is common in ovarian cancer, the disease most often spreads intraperitoneally; free-floating cells shed from the primary tumor are capable of implanting on any peritoneal surface. Any peritoneal fluid (ascites) found when the peritoneal cavity is opened should be aspirated and submitted for cytologic examination. In the absence of peritoneal fluid, washings should be taken by lavage of the peritoneal surfaces, including the undersurface of the diaphragm ( Fig. 11.8 ). Care should be taken to visualize and palpate all peritoneal surfaces, particularly the diaphragm, the surface of the liver, the lateral abdominal gutters, and the small and large bowel mesentery. The omentum should be removed because microscopic disease is often present in the omentum that is not obvious grossly. Recommended surgical therapy is presented in sequence in Table 11.11 . If the disease is limited to the pelvis, the primary tumor should be removed intact. All suspicious surfaces in the peritoneal cavity should be removed as biopsy specimens. This includes adhesions, which should be excised, not incised, because they often contain microscopic disease. Several studies have been performed to investigate the efficacy of “blind” peritoneal biopsies and routine retroperitoneal node dissections in the proper staging of early epithelial cancer of the ovary ( Table 11.12 ). Proper staging is important for treatment planning and for providing an accurate prognosis ( Table 11.13 ).
| FIGO Stage | Description |
|---|---|
| I | Tumor confined to ovaries or fallopian tube(s) |
| IA | Tumor limited to one ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings |
| IB | Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings |
| IC | Tumor limited to one or both ovaries or fallopian tubes, with any of the following:
|
| II | Tumor involves one or both ovaries or fallopian tubes with pelvic extension (below pelvic brim) or primary peritoneal cancer |
| IIA | Extension and/or implants on uterus and/or fallopian tubes and/or ovaries |
| IIB | Extension to other pelvic intraperitoneal tissues |
| III | Tumor involves one or both ovaries or fallopian tubes or primary peritoneal cancer with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes |
| IIIA | IIIA1: Positive retroperitoneal lymph nodes only (cytologically or histologically proven): IIIA1(i) Metastasis ≤10 mm in greatest dimension IIIA1(ii) Metastasis >10 mm in greatest dimension IIIA2: Microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes |
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis ≤2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis >2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ) |
| IV | Distant metastasis excluding peritoneal metastases
|

| Peritoneal cytologic examination Determination of extent of disease
Removal of all tumor possible (total abdominal hysterectomy and bilateral salpingo-oophorectomy) plus node sampling and omentectomy |
| Series | Stage I | Stage II | Stages III and IV | Total | |||
|---|---|---|---|---|---|---|---|
| Positive Lymphangiography | Positive Biopsy | Positive Lymphangiography | Positive Biopsy | Positive Lymphangiography | Positive Biopsy | ||
| Hanks and Bagshaw (1969) | 2/9 | — | 2/6 | — | 4/7 | — | 8/22 |
| Parker et al. (1974) | 3/13 | — | 2/29 | — | 12/27 | — | 17/69 |
| — | 5/26 | — | — | — | — | — | |
| 1/5 | — | 1/5 | — | — | 3/5 | 2/10 | |
| Buchsbaum et al. (1989) * | — | 4/95 | — | 8/41 | — | 7/46 | — |
| Burghardt (1991) | — | 1/20 | — | 4/7 | — | 51/78 | — |
| Total | 10/141 | 12/48 | 61/129 | ||||
* All patients had optimal carcinoma with metastatic lesions smaller than 3 cm.
| Stage | Patients ( n ) | 5-Year Survival (%) |
|---|---|---|
| Ia | 632 | 89 |
| Ib | 69 | 86 |
| Ic | 663 | 83 |
| IIa | 72 | 70 |
| IIb | 93 | 65 |
| IIc | 241 | 71 |
| IIIa | 128 | 46 |
| IIIb | 271 | 41 |
| IIIc | 2030 | 32 |
| IV | 626 | 18 |
Therapeutic Options for Primary Treatment
Borderline Malignant Epithelial Neoplasms
Over the past 4 decades, investigators have described a subset of epithelial ovarian tumors whose histologic and biologic features lie between those of clearly benign and frankly malignant ovarian neoplasms. These borderline malignant neoplasms account for approximately 15% of all epithelial ovarian cancers and share molecular characteristics with low-grade ovarian cancers. They have also been called proliferative cystadenomas and tumors of low malignant potential and are more completely discussed in Chapter 10 . Compared with invasive epithelial ovarian cancer, epithelial borderline tumors affect a younger population and may have a 10-year survival rate approaching 95% (see Chapter 10 ). Although symptomatic recurrence and death may rarely develop as late as 20 years after therapy, these neoplasms are correctly labeled as being of low malignant potential. Most gynecologic oncologists recommend conservative therapy, especially in patients who are desirous of further childbearing and have stage IA disease (see Chapter 10 ). We have found surgical excision of disease the most effective therapy and when necessary have used repeated explorations, reserving chemotherapy for patients who develop ascites or whose tumor changes histologic features or demonstrates rapid growth ( Fig. 11.9 ).
Treatment of Malignant Epithelial Neoplasms
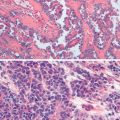
The most common epithelial cancers of the ovary are histologically categorized as serous, mucinous, endometrioid, and clear cell (mesonephroid) types ( Figs. 11.10 to 11.13 ). Most large studies demonstrate that these different histologic types have similar outcomes with existing therapy, stage for stage and grade for grade. Mucinous and endometrioid subtypes are more commonly found in earlier stages. There are ongoing studies to investigate targeted therapies in certain cell types, most notably mucinous and clear cell cancers. Based on our current data, however, prognosis, survival, and therapy for these various forms of epithelial cancer will be considered collectively.
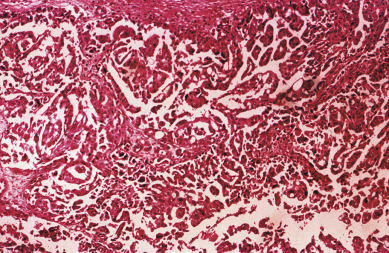
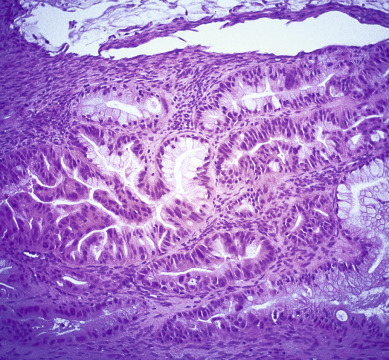
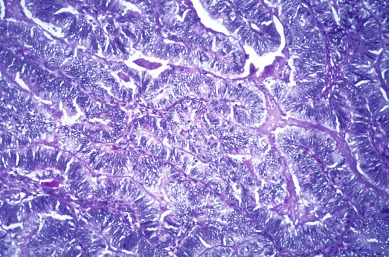
The unifocal theory of ovarian epithelial cancer growth suggests that the disease initially grows locally, invading the capsule and mesovarium, and then invades adjacent organs by local extension and lymphatic spread. Cells on the surface exfoliate into the peritoneal cavity, where they circulate and implant on free peritoneal surfaces. Local and regional lymphatic metastasis may involve the uterus, fallopian tubes, and pelvic and aortic lymph nodes.
Woodruff suggested a multifocal mechanism of disease spread, whereby the entire coelomic epithelium can give rise to this lesion after exposure to carcinogenic agents that enter the peritoneal cavity from the vagina through the fallopian tubes. Indeed, the lesion could then originate in a multifocal distribution, “like a measles rash,” over large portions of the coelomic epithelium. Although the early precursors leading to advanced ovarian cancer are incompletely understood, more current molecular evidence favors the unifocal origin theory.
In the past decade, as risk-reducing salpingo-oophorectomy has become more common, a moderate body of literature suggests that a significant percentage of “ovarian cancers” may actually arise in the fallopian tube fimbria with metastasis to the ovaries. Kindelberger and colleagues have reported a potential causal relationship based on identification of tubal intraepithelial carcinomas (TIC) and analysis of p53 mutations therein.
Regardless of origin, the most important prognostic variable for each patient is the extent, or “stage,” of disease. A staging system has been devised that allows treatment results in patients with similar prognostic factors to be compared among different institutions. Survival is affected by the cancer stage, grade of differentiation, gross findings at surgery ( Table 11.14 ), amount of residual tumor after surgery, and additional treatment required.
|
Stages IA, IB, and IC
Careful surgical staging is critical in the management of stage I invasive ovarian cancer and should include bilateral salpingo-oophorectomy, hysterectomy, omentectomy, and pelvic and aortic lymph node sampling, with peritoneal biopsies and washings. Accurate staging forms the cornerstone of clinical decision-making regarding the type and duration of adjuvant therapy.
Pelvic and aortic lymph nodes may be involved 10% to 20% of the time in apparent stage I disease, and lymphadenectomy is an important diagnostic and therapeutic procedure. Baiocchi and associates reviewed their experience in 242 women who had pelvic and aortic lymphadenectomy in whom cancer was apparently confined to the ovaries (stage I), and nodal metastasis was found in 32 patients (13%). Patients with serous adenocarcinoma had the highest incidence of node metastasis (25%). Those with grade III lesions had 39% metastasis compared with 6% with grade I and grade II cancers. There were 33 women with low malignant potential tumors, and seven (21%) had nodal metastasis. When only one to three nodes were involved, metastases were usually ipsilateral, but these patients could also have metastases to the common iliac or aortic nodes. Positive aortic nodes were also found in the absence of positive pelvic nodes. Accumulated reports of lymph node metastases in clinical stage I ovarian cancer patients are shown in Table 11.15 . Without thorough surgical staging, occult metastasis may be present and missed, leading to inadequate subsequent therapy. Many studies have addressed this item ( Table 11.16 ).
| Author | Patients |
|---|---|
| Di Re | 16/128 |
| Benedetti–Panici | 5/25 |
| Wu | 1/7 |
| Burghardt et al. | 9/37 |
| Petru | 9/40 |
| Knapp and Friedman | 5/26 |
| Tsuruchi | 1/51 |
| Chen et al. | 3/11 |
| Lanza | 3/11 |
| Carnino | 2/47 |
| Baiocchi | 32/242 |
| 86/625 (13.7%) |
| Localization | Frequency (%) | Range (%) |
|---|---|---|
| Peritoneal cytology | 22 | 10–46 |
| Diaphragm | 9 | 0–44 |
| Omentum | 5 | 0.7 |
| Pelvic nodes | 8 | 0–20 |
| Paraaortic nodes | 12 | 0–25 |
| Pelvic peritoneum | 9 | 6–10 |
| Abdominal peritoneum | 8 | 7–9 |
| Bowel mesentery-serosa | 6 | 3–13 |
The use of adjuvant therapy and its role in stage I ovarian cancer continues to be investigated. Through three GOG studies in early-stage (I and II) ovarian cancer, the use of platinum combination therapy became the preferred regimen as it demonstrated better survival outcomes and an improved, although not statistically significant, toxicity profile over intraperitoneal (IP) colloid 32P.
European investigators have also reported their experience with platinum-based therapy in early-stage ovarian cancer. The European gynecologic groups reported a combined analysis of the International Collaborative Ovarian Neoplasm (ICON) 1 and the Adjuvant Chemotherapy In Ovarian Neoplasm (ACTION) trials. More than 900 patients with early-stage ovarian cancer received either platinum-based adjuvant chemotherapy or observation until chemotherapy was indicated. After a median follow-up period of more than 4 years, the improved overall survival (OS) (82% chemotherapy vs. 74% observation) and recurrence-free survival (76% vs. 65%, respectively) rates at 5 years favored treatment with platinum-based therapy. Although not all patients had undergone comprehensive staging, a subgroup analysis of these patients was reported. In another European Organization for Research and Treatment of Cancer (EORTC)-ACTION trial of 448 patients, the authors noted that the benefit of chemotherapy was limited to patients who lacked comprehensive surgical staging, questioning the necessity of adjuvant therapy in the patient with well-staged early ovarian carcinoma.
The next evolution of GOG trials involved the addition of paclitaxel to a platinum-based regimen because the treatment protocols for advanced-stage disease had demonstrated improved survival by replacing cyclophosphamide with paclitaxel. GOG 157 evaluated carboplatin (AUC 7.5) and paclitaxel (175 mg/m 2 ) in high-risk, early-stage disease, randomly assigning patients to three versus six chemotherapy cycles. Over a 3-year interval, 457 patients were enrolled and evaluated after a median follow-up period of 6.8 years. Although the recurrence rate was 24% lower with the six cycles ( P = 0.18), OS was similar for the two arms (hazard ratio [HR], 1.02). The study concluded that the additional three cycles contributed to increased toxicity (11% grade III/IV neurotoxicity vs. 2% with only three cycles) without an OS advantage. Building on the hypothesis that low-dose paclitaxel may have antiangiogenesis activity, GOG 175 tested whether the addition of 24 weekly paclitaxel treatments (60 mg/m 2 ) to the initial three cycles of carboplatin and paclitaxel would improve outcomes. Although the recurrence rate was 20% lower in patients receiving extended paclitaxel, the result was not statistically significant, and there was no appreciable difference in OS.
In the young woman with stage IA disease who is desirous of further childbearing, unilateral salpingo-oophorectomy may be associated with minimal increased risk of recurrence, provided a careful staging procedure is performed and due consideration is given to grade and apparent self-containment of the neoplasm. After conservative treatment for invasive ovarian cancer, term delivery rates have been reported as high as 30%, and successful pregnancy outcomes have been reported after adjuvant chemotherapy. Conservative surgery is generally not recommended for patients with clear cell or carcinosarcoma or grade III tumors and when disease is present outside the ovaries. A careful discussion regarding the risks and benefits of this approach is essential because Maltaris and colleagues reported that 12% of patients undergoing fertility-sparing ovarian cancer surgery experienced recurrence, and 4% of patients died from their disease.
In the management of stage I ovarian cancer, the physician must weigh the possible benefits of adjuvant chemotherapy against the risks. It appears reasonable to not recommend adjuvant therapy for patients with stage IA, IB, grade 1 and 2 lesions who have been comprehensively staged. Patients with stage I, grade 3 and stage IC disease present a more difficult problem because the incidence of recurrence in this group approaches 50% in some series. We favor treatment with platinum-based combination chemotherapy even though no clear data show a survival advantage over single-agent therapy. Because combination therapy is our best treatment for metastatic disease, it is difficult to withhold the apparent best therapy from a group of patients who may have the best situation for a chemotherapy “cure.” In addition, this group is usually younger and better able to tolerate combination chemotherapy.
Stages IIA, IIB, and IIC
In most institutions, the therapy of choice for stage IIA and stage IIB disease is staging surgery followed by platinum-based combination chemotherapy. Although historically the role of pelvic and abdominal irradiation and IP 32P has been described, the radioisotope and irradiation treatment approaches have faded from frontline therapy for ovarian carcinoma. Careful surgical staging is essential to successful treatment planning. A combined analysis of GOG 95 and 157 revealed that the stage II patients represented a disproportionate percentage of the recurrences. The GOG now includes stage II patients in their clinical trials of patients with advanced-stage disease. Outside of a clinical trial, patients with stage II disease should be managed in a similar fashion to optimally debulked stage III disease.
Stage III
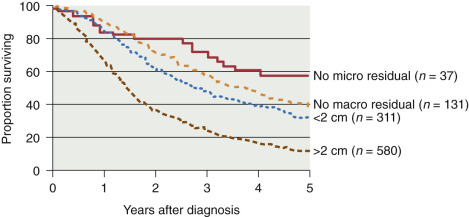
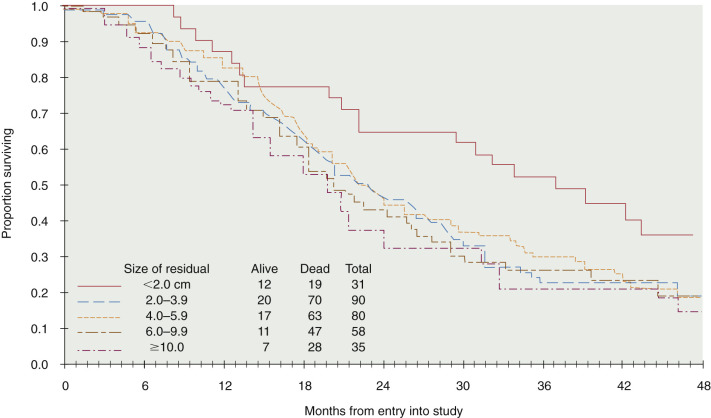
In addition to surgical staging, every reasonable effort should be made to remove all visible metastatic tumors. Retrospective studies have strongly suggested that the survival rate in patients with stage III disease is related to the amount of residual tumor after surgery, such that patients with no macroscopic residual tumor appear to have the best prognosis after primary chemotherapy ( Table 11.17 and Fig. 11.14 ). Most centers prefer combination platinum-based chemotherapy for this group of patients because of the excellent response rates reported in the literature (see later section on combination chemotherapy and IP therapy).
| Author | Patients ( n ) | Optimal (<2 cm) | Suboptimal (>2 cm) |
|---|---|---|---|
| Griffiths et al. | 102 | 28 | 11 |
| Wharton et al. | 104 | 27 | 15 |
| Hacker et al. | 47 | 22 | 6 |
| Sutton et al. | 56 | 39 | 22 |
The duration of multiple-agent therapy is usually six cycles. If after primary chemotherapy the patient has no clinical evidence of disease (clinical complete response), a second-look procedure was often considered in the past to ascertain the presence of subclinical residual disease. However, a secondary data analysis of GOG 158 suggested no apparent outcome advantage from second-look surgery. For the most part, second-look surgery is no longer recommended.
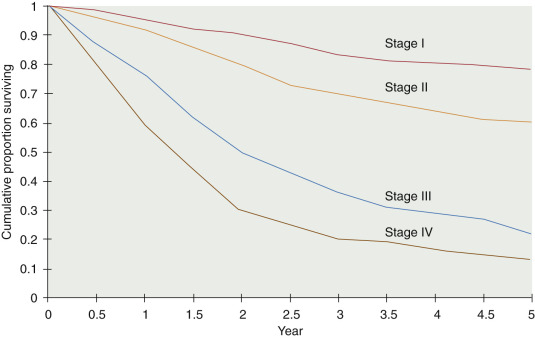
Stage IV
The ideal management of stage IV disease is to remove as much cancer as possible and to administer chemotherapy after surgery. The role and benefit of surgical cytoreduction in these patients is detailed in the next section. The OS is worse for this group of patients than for those of other stages, as expected ( Fig. 11.15 ).
Maximal Surgical Effort
The goal of primary surgery for advanced ovarian cancer should be to remove all visible tumor and to use all reasonable surgical means to do so ( Fig. 11.16 ). In the 1970s, Griffiths and coworkers evaluated the importance of postsurgical tumor residual by using a multiple linear regression equation with survival as the dependent variable to control simultaneously for the multiple therapeutic and biologic factors that contribute to the ultimate outcome in the individual patient. The most important factors proved to be histologic grade of the tumor and size of the largest residual mass after primary surgery. In Griffiths et al.’s original study, the operation itself contributed nothing to survival unless it effected reduction in the size of the largest residual tumor mass below the limit of 1.6 cm.
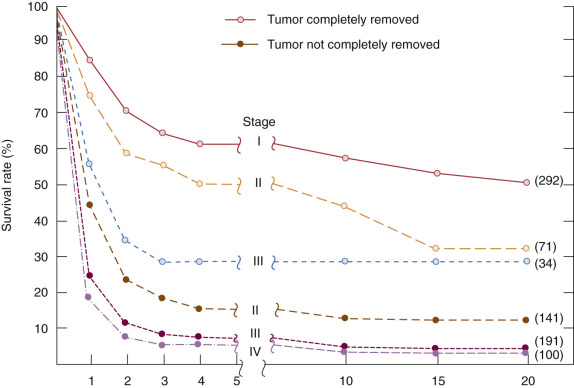
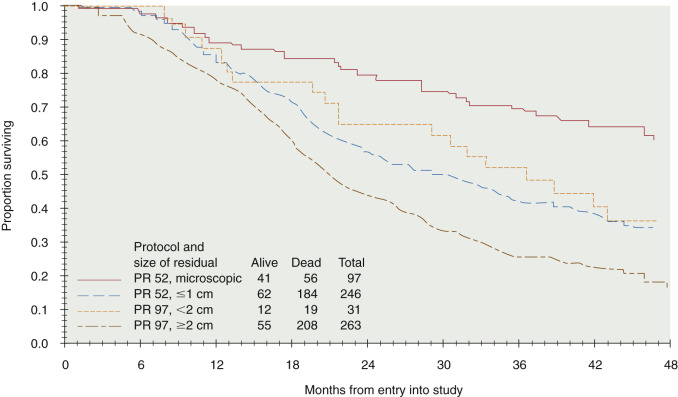
Cytoreductive or “debulking” procedures have gained considerable attention in the management of ovarian cancer. The concept is to reduce the residual tumor burden to a level at which subsequent chemotherapy can be most effective. A careful dissection by a well-trained gynecologic oncologist can often remove disease that on first impression appear to be unresectable ( Fig. 11.17 ). Removal of large ovarian masses and omental involvement may reduce the tumor burden by 80% to 99%. The theoretical value of debulking procedures lies in reducing the number of tumor cells and the advantage this affords to adjuvant therapy. This is especially relevant in bulky chemosensitive solid tumors such as ovarian cancer, in which removal of large numbers of cells in the resting phase (G0) may propel the residual cells into the more vulnerable proliferating phases. Several careful retrospective studies have repeatedly demonstrated improved survival rates in patients who can be surgically reduced to minimal tumor burden. The GOG has attempted to better define primary cytoreductive surgery with a detailed analysis of the results of surgery in patients with advanced disease. Their initial study compared survival of the patients with stage III disease who were found at surgery to have abdominal disease of 1 cm or smaller with that of patients found to have disease larger than 1 cm but whose tumors were surgically cytoreduced to 1 cm or less. If surgery was the only important factor, survival should have been equivalent in both groups. This was not the case. Patients found to have small-volume disease survived longer than did patients who had cytoreduction to small-volume disease at surgery, suggesting that the tumor biology also carries prognostic significance. In a second study, GOG investigators evaluated the effect of the diameter of the largest residual disease on survival in patients with suboptimal cytoreduction. They demonstrated that cytoreduction so that the largest residual mass was 2 cm or less resulted in a significant survival benefit, but all residual diameters larger than 2 cm had equivalent survival ( Fig. 11.18 ). Therefore, unless the mass could be cytoreduced to 2 cm or less, residual diameter did not influence survival. In evaluating optimal and suboptimal cytoreduction, these GOG investigators showed that three distinct groups emerged: microscopic (no visible) residual, residual disease of less than 2 cm, and residual disease of greater than 2 cm ( Fig. 11.19 ). It is clear from these studies that whereas patients with microscopic disease had a 4-year survival rate of about 60%, patients with gross disease 2 cm or less had a 4-year survival rate of 35%. However, patients whose disease could not be cytoreduced to 2 cm or less had a 4-year survival rate of less than 20%. Most striking, however, was the failure of cytoreductive surgery to improve survival unless the largest diameter of residual disease was 2 cm or less. The effect of maximal cytoreductive surgery can be seen in the percentage of negative second-look procedures ( Table 11.18 ).
| Author (Year) | Negative Second Look (%) | ||
|---|---|---|---|
| No Residual | Optimal Residual | Suboptimal Residual | |
| Barnhill (1984) | 67 | 61 | 14 |
| Cain (1986) | 76 | 50 | 28 |
| Smirz (1985) | 75 | — | 25 |
| Webb (1982) | 95 | 36 | 20 |
| Podratz (1983) | 82 | 44 | 33 |
| Curry (1981) | 79 | 45 | 22 |
| Dauplat (1983) | 100 | 100 | 40 |
| 75 | 45 | 25 | |
| Mean | 81 | 52 | 23 |
An inverse correlation between the amount of residual disease after surgery and OS has been consistently associated. In 2002, Bristow and associates published a meta-analysis of published studies at the time, in which the impact of cytoreduction was clearly demonstrated. By comparing currently available studies of advanced ovarian cancer patients undergoing surgical cytoreduction, they demonstrated that for each 10% increase in the proportion of patients undergoing optimal cytoreduction, the median survival rate increased by 5.5% to 6%. Although the current accepted definition of “optimal cytoreduction” is no visible residual tumor smaller than 1 cm in greatest dimension, the best reported outcomes occur in patients who have complete cytoreduction or no visible residual disease.
In an effort to remove visible tumor deposits and achieve optimal cytoreduction, gynecologic oncologists frequently perform radical surgical procedures beyond those required for standard staging. Eisenkop and associates described 163 consecutive patients assigned to stage IIIC and stage IV. Complete cytoreduction could be performed in 86% of patients using an array of radical procedures. The overall median survival time was 54 months, but it was 62 months in those whose tumors were optimally debulked. Aletti and colleagues reported that for patients cytoreduced to low-volume residual disease, their favorable survival was directly associated with the need for radical surgery. Two sites that often require radical operations to clear are tumor metastases to the bowel and to the upper abdomen. Multiple investigators have reported the utility and safety of large or small bowel resection, which may be necessary in up to 50% of cases to achieve optimal cytoreduction and has been associated with a subsequent improvement in survival. Upper abdominal cytoreduction has also been shown to be safe and effective but may require additional training or assistance. Cliby and colleagues reported procedures such as liver resection, splenectomy, and diaphragm resection to be possible and associated with an acceptable morbidity. Chi and coinvestigators demonstrated that the addition of upper abdominal procedures to achieve optimal cytoreduction resulted in a median survival time longer than 5 years, which was comparable to that of patients who did not require these extensive procedures for optimal cytoreduction. Improvements in surgical cytoreduction have developed in consort with additional advances in intensive care and postoperative care, allowing additional benefits for our ovarian cancer patients.
The role of cytoreductive surgery in stage IV cancers has been investigated. Several studies have suggested that optimal debulking can be performed in many of these patients with beneficial effect. Liu and associates described 47 patients with stage IV cancers, of which 14 (30%) were optimally debulked to less than 2 cm residual. The median survival time was 37 months in the patients in whom the cancer was optimally debulked compared with 17 months in the patients in whom the cancer was suboptimally debulked. A study by Chi and colleagues identified 92 patients; optimal debulking was achieved in 45% with a median survival time of 40 months compared with 18 months for suboptimal debulking. A recent ancillary study by the GOG demonstrated that the ability to achieve cytoreduction to less than 5 cm residual disease in stage IV patients rendered some survival advantage, although the most favorable subgroup was patients reduced to no visible residual disease. Aletti and colleagues also demonstrated that using radical procedures to perform cytoreduction to no visible residual disease resulted in median survival times longer than 3 years in stage IV patients. For patients known or suspected to have thoracic metastases, the use of video-assisted thoracic surgery (VATS) can be diagnostic, potentially therapeutic, or both. Positive findings on VATS may allow for intrathoracic cytoreduction in select cases or may indicate which patients are best served with neoadjuvant chemotherapy as a result of unresectable pleural disease.
Even though cytoreductive surgery consistently appears to have therapeutic value, controversy continues. The primary unresolved issue is whether the poor prognosis with bulky disease is caused by the presence of increased tumor burden (in which case cytoreductive surgery is of potential benefit) or whether it is associated with differences in tumor biology or a decreased sensitivity to chemotherapeutic regimens. (If these possibilities are indeed the case, cytoreductive surgery is not likely to have a major impact on survival.) The implication is that patients who have disease that can be cytoreduced are a select group with good prognoses on the basis of factors independent of the surgery. Furthermore, if chemotherapy is delayed because of complications of surgery, this may have a deleterious effect on long-term survival. The percentage of patients with advanced ovarian cancer who can effectively undergo cytoreductive surgery seems to range from 43% to 87%, depending on the investigators reporting. This difference may not only reflect the individual skills of the surgeons but also may be influenced by different referral patterns and other selection factors. Neoadjuvant chemotherapy has been investigated as a means by which to address this issue; these studies are described in the subsequent section.
The role of lymphadenectomy in patients with advanced disease continues to be debated. Lymph node involvement is common in patients with advanced disease (>50%); the question is whether lymphadenectomy improves survival. It has been suggested by some that metastases in lymph nodes do not respond well to chemotherapy, and therefore these potential metastatic sites should be removed. Opponents state that recurrence most frequently occurs in the peritoneal cavity, and therefore lymph node status has little impact on the overall disease course. Several studies have come to somewhat different conclusions. Parazzini and associates evaluated 456 women with stage III or IV disease entered in a prospective randomized chemotherapy trial. There were 161 patients with positive nodes. They noted grade 3 tumors to have a greater number with positive nodes compared with grade 1 or grade 2 tumors. This was also true of stage IV compared with stage III cancer. They did not find a difference in survival between those with positive nodes and those with negative nodes. Scarabelli and colleagues evaluated lymph node status in 98 patients with stage IIIC to IV disease who had no gross residual disease after surgery compared with 44 patients who did not undergo lymphadenectomy. Survival was significantly improved if lymphadenectomy was done (Cox analysis). In 2005, Panici and coinvestigators reported their prospective multicenter analysis of 427 advanced ovarian cancer patients randomly assigned to lymphadenectomy or removal of bulky nodes only. Although progression-free survival (PFS) was improved in patients undergoing lymphadenectomy, OS was not different. The 2005 retrospective analysis of 889 patients enrolled in the Scottish Randomised Trial in Ovarian Cancer (SCOTROC-1) was conducted. The analysis demonstrated that optimal debulking (<2 cm) was more commonly achieved in patients accrued from the United States, Europe, and Australia than in patients recruited from the United Kingdom (71% vs. 58%). Comparison of the UK and non-UK patients who had no visible residual disease demonstrated a better survival for the non-UK patients, and the authors attributed this to the fact that non-UK patients more commonly underwent primary lymphadenectomy, suggesting a potential therapeutic benefit. It has been our practice to do pelvic and aortic lymphadenectomy routinely if the patient’s tumor can be optimally debulked. Benefits of lymphadenectomy in patients with bulky residual disease remain questionable. It currently seems appropriate that patients with a diagnosis of advanced ovarian cancer should undergo resection of all masses when technically feasible.
Because a suboptimal surgical effort confers no survival benefit and adds only postoperative morbidity, accurate methods to determine which patients could be successfully resected would be useful. Both preoperative imaging and initial laparoscopy have been investigated as methods to determine the likelihood of success. Most imaging series have used preoperative CT scan to assess factors that could predict an optimal cytoreduction. Although sets of radiographic risk factors have been described, these have had poor predictive value because the majority of patients (62%–86%) identified as having “poor prognostic indicators” have been able to undergo optimal cytoreduction. Similarly, laparoscopy in advanced ovarian cancer has limitations in terms of how complete an assessment of the IP space is possible, and metastases at port sites have been reported. Wang and associates reviewed the literature to determine risk factors that may contribute to early recurrence at the port site. Of the gynecologic cancers, ovarian cancer was the most common malignant neoplasm with subsequent port site metastasis. Metastasis to the port site was more common if ascites and IP carcinomatosis were present. The earliest time from laparoscopy to port site metastasis was 8 days; however, the clinical significance of port site metastases is unclear because they often clinically resolve with systemic therapy.
Whether a suboptimal primary surgery can be improved upon by subsequent surgical cytoreduction after chemotherapy is controversial. The EORTC group reported its initial experience with interval debulking surgery in patients with advanced ovarian carcinoma after unsuccessful primary cytoreductive surgery. Patients received three courses of cisplatin and cyclophosphamide and were then randomly assigned to receive interval debulking surgery or no further surgery. All patients received six courses of chemotherapy. There were 278 patients evaluated, and the median survival rate was 26% versus 20% (surgery vs. no surgery; P = 0.012). Debulking surgery was an independent prognostic factor in multivariate analysis. After adjustment for all prognostic factors, surgery reduced the risk of death by 33% ( P = 0.008). GOG investigators conducted a similar study. This study (GOG 152) enrolled 550 patients, all of whom had initial primary surgery by a gynecologic oncologist. Patients who were suboptimally debulked (residual tumor >1 cm) received three cycles of paclitaxel plus cisplatin. Patients were randomized to continued chemotherapy or to a secondary surgical cytoreduction and then continued chemotherapy. Neither the PFS nor the relative risk of death was improved with the additional interval surgery. The authors speculate that the patients in the GOG study may also have undergone more aggressive primary surgery than the patients in the EORTC study, and this may have accounted for the difference in results. Most important, one could summarize these two studies by concluding that patients with advanced ovarian cancer are deserving of at least one maximal effort at cytoreduction, preferably by a gynecologic oncologist.
Although a maximal primary surgical effort appears to be a cornerstone of potential long-term survival, the timing of the surgical effort remains a focus of debate. In patients with borderline performance status, based on age, compromising medical disease, and extensive effusions (especially pleural or pericardial), and in patients with extensive abdominal disease, unlikely to be effectively debulked, strong consideration should be given to initiating therapy with neoadjuvant chemotherapy. After two to four cycles, a good clinical response to chemotherapy may provide an opportunity for an effective surgical debulking with an acceptably low complication rate. Before initiating chemotherapy, the diagnosis of ovarian, tubal, or peritoneal cancer should be confirmed by cytology or minimally invasive surgery. Neoadjuvant chemotherapy treatment (followed by surgical debulking) results have been reported in small retrospective reports of between 20 and 90 patients and generally report survival outcomes similar to those of patients who have been suboptimally cytoreduced. A meta-analysis of these studies by Bristow et al. and Chi et al. demonstrated that the use of neoadjuvant chemotherapy was associated with a reduction in survival of approximately 4 months with every preoperative cycle, implying that surgical intervention should be performed as soon as is feasible. However, Vergote and colleagues have reported results from the large randomized EORTC noninferiority trial of patients with advanced ovarian cancer treated with primary surgery followed by chemotherapy versus neoadjuvant chemotherapy for three cycles followed by surgical cytoreduction and three additional cycles of chemotherapy. Complete resection of all macroscopic disease was the strongest predictor of OS. They demonstrated comparable survival outcomes (~29 months in both arms), and overall the results favored neoadjuvant chemotherapy given the lower surgical morbidity compared with the primary surgery group. Another trial evaluating the role of neoadjuvant chemotherapy, Chemotherapy Or Upfront Surgery in Ovarian Cancer Patients (CHORUS), randomized women with stage III or IV ovarian cancer to receive upfront surgery followed by six cycles of chemotherapy (carboplatin and paclitaxel) or three cycles of chemotherapy, surgery, and then three additional cycles of completion chemotherapy. A total of 550 women were evaluated, and the OS time was 22.6 months in the primary surgery group compared with 24.1 months in the primary chemotherapy group. The authors also reported a lower rate of grade 3 or 4 postoperative adverse events and deaths in the primary chemotherapy group, concluding that the primary chemotherapy approach is noninferior to the primary surgery approach. Although the neoadjuvant approach is a reasonable option for some patients, it is important to recognize several criticisms to the study. Both studies reported low rates of primary optimal cytoreduction (~40% in both studies) and low median OS compared with other contemporary ovarian cancer trials. Therefore, neoadjuvant chemotherapy has not been widely adopted as an equivalent primary treatment option in the United States, and further studies are ongoing.
Role of Radiation Therapy
With improvements in chemotherapy and surgery for ovarian cancer, radiation therapy rarely plays a role in primary therapy. The systemic pattern of spread makes effective radiation therapy difficult, and with bulky residual disease, radiation therapy is particularly ineffective. Studies by the GOG and others demonstrated the extent to which the addition of radiation did not improve outcomes even before the current era of highly active chemotherapy. Some special problems are listed in Table 11.19 . The entire abdomen must be considered at risk; therefore, the volume that must be irradiated is large, resulting in multiple limitations for the radiotherapist. However, consideration of radiotherapy for specific histologies and isolated recurrences is being further studied. Dose restrictions are listed in Table 11.20 .
|
|
Radioisotopes
Numerous trials have been conducted comparing IP 32P with or without pelvic radiation with whole abdominal radiation or single-agent chemotherapy in various clinical settings of ovarian cancer. Because IP 32P has failed to demonstrate improved outcomes, can be associated with increased complications, and is “technique intensive,” it is rarely considered for contemporary treatment planning.
Chemotherapy
The evolution of chemotherapy agents for advanced ovarian cancer over the past 30 years has been significant. Ovarian cancer was one of the first solid malignant tumors demonstrating responsiveness to chemotherapy. Effectiveness of the various chemotherapy treatments has been measured by response rates (usually complete plus partial responses), negative second-look rates, PFS, and OS. All of these outcome measures can be subject to error. Even survival outcomes can be compromised by the fact that many patients receive a number of variously active chemotherapy treatments over their treatment history.
The earliest agents used for the treatment of ovarian carcinoma (1970–1980) were predominantly the alkylating agents melphalan (also called phenylalanine mustard, Alkeran, L-PAM, and L-sarcolysin), cyclophosphamide, chlorambucil, and thiotepa. Response rates were usually reported in the 20% to 60% range, but median survival rates for patients with advanced ovarian cancer were often in the range of 10 to 18 months, quite inferior to most outcomes from today’s clinical trials. Antimetabolites, such as 5-fluorouracil and methotrexate, were also used in many of the earlier trials, especially in combination with alkylating agents. The contemporary use of these agents in epithelial ovarian cancer is rare to nonexistent.
The late 1970s and 1980s saw the introduction of combination chemotherapy regimens, such as Hexa CAF (hexamethylmelamine, cyclophosphamide, doxorubicin, and 5-fluorouracil) and CAP (cyclophosphamide, doxorubicin, and cisplatin). By the early 1980s, combination therapy was the standard treatment for most patients. The 1980s also witnessed the introduction of cisplatin and later carboplatin. The introduction of these platinum compounds increased response rates to the 50% to 80% range and increased median survival periods to the 12- to 30-month range in most studies. The wide range was often attributable to select patient populations, with the “suboptimal” debulked patients having the 12- to 18-month survival times and the “optimal” debulked patients having the 18- to 30-month survival times. The platinum compounds have remained an integral component of treatment to this day.
The 1990s saw the introduction of paclitaxel, an agent first extracted from the stripped bark of the Pacific yew tree, Taxus brevifolia. Paclitaxel, now chemically synthesized, demonstrated a new mechanism of action by promoting microtubular assembly and stabilizing tubulin polymer formation, thus inhibiting rapidly dividing cells from completing the mitotic process. The initial single-agent paclitaxel response rates in patients with refractory ovarian cancer were in the 25% to 35% range. Contemporary drug development continues to look at new formulations of the taxane group. Docetaxel in several studies has shown equivalent efficacy to paclitaxel with a different side effect profile, and other modified taxanes such as Abraxane and CT-2103 have been evaluated .
The past 2 decades have also seen the introduction of additional cytotoxic agents with demonstrated activity in ovarian cancer, including topotecan, a topoisomerase I-inhibitor; a pegylated liposomal encapsulated form of doxorubicin; and gemcitabine. These three drugs were tested in the frontline setting by the GOG 182/ICON-5 clinical trials, discussed later.
For the past decade, there has been considerable interest in developing agents targeting specific molecular targets. Two important categories of targeted therapies in ovarian cancer have been angiogenesis inhibitors, of which bevacizumab has been most widely studied, and poly(ADP-ribose) polymerase (PARP) inhibitors. Immune therapies continue to be in development and will continue to be a therapeutic focus in the next decade.
Clinical Trials
The relatively low rates of response to most single agents stimulated investigators to search for combination schedules. In the “modern” era, platinum-based combinations have proved to be the most successful. Pertinent clinical trials relating to early-stage disease are discussed earlier in this chapter, and the following discussion focuses on trials for advanced ovarian cancer.
Development of ovarian cancer combination chemotherapy through the 1980s and 1990s saw transition from doxorubicin–cyclophosphamide (AC), to cyclophosphamide–doxorubicin–cisplatin (CAP), to cyclophosphamide-platinum, and finally to taxane–platinum as the evolving standard of care. The trials performed by the GOG as well as European investigators were primarily responsible to developing these standards. In 1986, GOG 47 reported results comparing CAP to AC, with improved response rate, response duration, and PFS but not OS in the whole cohort. Subgroup analysis of patients with measurable disease (227 of 440 assessable patients) did show improved OS, and the potential advantage of incorporating platinum therapy was recognized. GOG 52 evaluated CAP versus cyclophosphamide–cisplatin alone in optimally cytoreduced patients and demonstrated equivalent PFS and OS endpoints. With less toxicity by withholding doxorubicin, cyclophosphamide–cisplatin became the “standard” arm for many clinical trials in the late 1980s and early 1990s. Before abandoning doxorubicin completely, four trials were considered in a meta-analysis that specifically evaluated the benefit of doxorubicin in ovarian cancer. Considering only pathologic complete responses, the study showed a constant small benefit for CAP, highest in the North-West Oncology Group (GONO) and Danish Ovarian Cancer Group (DACOVA) studies. By pulling together these data in a meta-analysis, it was possible to detect a statistically significant benefit of pathologic complete responses (6%) and OS (7%) with CAP. However, because in three trials the dose intensity was greater for CAP than for cyclophosphamide–cisplatin, to what extent the benefit of CAP is from greater dose intensity or from doxorubicin itself remains unsolved.
In the 1990s, paclitaxel became available on trial, and GOG 132 evaluated 614 patients with suboptimally debulked cancer who were treated in a three-arm protocol comparing cisplatin alone, paclitaxel alone, and a combination of the two drugs. Neither PFS nor OS was different among the three arms, but significant crossover to the other drug in the single-drug arms muddled somewhat the clarity of the outcome data. Some interpreted the results of this study to indicate that a platinum agent should be a component of primary therapy. With some evidence for efficacy, the paclitaxel–cisplatin combination was compared to the existing standard, cyclophosphamide–cisplatin. The GOG 111 study randomized 386 patients with large-volume disease to paclitaxel–cisplatin versus cyclophosphamide–cisplatin. In the paclitaxel arm, administration of paclitaxel before cisplatin was important to optimize response and minimize toxicity. In terms of therapeutic efficacy, the cisplatin–paclitaxel arm produced a significantly greater overall response rate (73% vs. 60%) and clinical complete response rate, but the frequency of pathologic complete response was similar between the two arms. The paclitaxel arm showed significant improvements in PFS (18 vs. 13 months) and OS (38 vs. 24 months). The risk of progression was 32% lower among those treated with the paclitaxel regimen, and the risk of death was 39% lower ( Fig. 11.20 ). A European-Canadian Intergroup trial (OV-10) had a study design similar to GOG 111, testing the replacement of cyclophosphamide with paclitaxel. This study included patients with optimal and suboptimal stage IB, IIB, III, and IV. Again, the clinical response rate was superior for the paclitaxel arm (59% vs. 45%), with a subsequent 25% reduction in the rate of death. The combination of paclitaxel and cisplatin became the standard for combination first-line chemotherapy for the treatment of epithelial ovarian carcinoma.
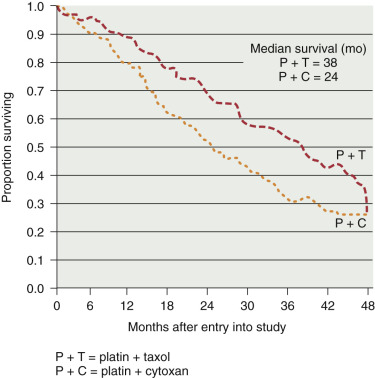
When combined with cisplatin, paclitaxel requires an extended infusion interval (24 hours) to prevent unacceptable neuropathy. This administration schedule was inconvenient, prompting many community providers to replace cisplatin with carboplatin, with which paclitaxel did not require this extended infusion. Several groups conducted equivalency trials to compare paclitaxel (175–185 mg/m 2 ) and carboplatin (AUC 5–7.5) with the standard cisplatin–paclitaxel regimen. The GOG conducted GOG 158 on the optimal (<1 cm) patient population as a noninferiority trial. The relative risk (RR) of progression on the paclitaxel plus carboplatin arm was equivalent (RR = 0.88; 95% CI, 0.–-1.03), and toxicity was greater on the paclitaxel plus cisplatin arm. Similar findings regarding therapeutic equivalence and toxicity preference for carboplatin over cisplatin were reported by the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Ovarian Cancer Study Group. The combination of intravenous (IV) paclitaxel and carboplatin replaced paclitaxel and cisplatin as the standard first-line chemotherapy for ovarian cancer.
Care must always be taken in interpreting response rates as a correlate for survival rates. Too often a chemotherapeutic regimen will produce an excellent response rate but not affect the OS rate. Therefore, the clinician must await longer term studies of combination chemotherapy to accurately understand its impact on survival of patients. Omura and colleagues reported a sobering analysis of two large GOG studies of multiagent chemotherapy in epithelial ovarian cancer. In this analysis of 726 women with stage III or stage IV disease, excellent follow-up had been obtained. The authors concluded that the impact of chemotherapy to date had been modest. Fewer than 10% of their patients were progression free at 5 years, and late failures continued to occur, even beyond 7 years. Sutton and coworkers reported a 7% disease-free survival rate at 10 years. Unfortunately, the superiority of any particular combination of chemotherapeutic agents as reflected by statistically significant improvement in long-term survival remains unproved. Although carboplatin appears to be the most active agent in epithelial cancer of the ovary, there is still controversy that combining it with other agents improves outcome.
In vitro testing of chemotherapy resistance or sensitivity has been investigated for at least 3 decades without clarification of its role in either primary or recurrent disease. Multiple technologies are currently in use, and there is a need to identify the relative value of this testing modality.
Dose-intense Chemotherapy
Dose intensity refers to the amount of chemotherapy to which a cancer is exposed per unit of time; this is generally assumed to be reflected by the dose of drug in milligrams per square meter of body surface area per unit of time. The theory is well grounded in preclinical work showing that as the concentration of drug in culture medium increases, the fraction of surviving cancer cells decreases logarithmically. The cell kill for a particular drug frequently follows a sigmoidal curve, indicating a point at which continued dose escalation will yield little improvement in results. To determine whether dose intensity is important clinically, randomized trials are needed. The GOG did design such a trial in patients with large-volume, advanced disease. A total of 458 patients were entered into the study and were randomly assigned to low-dose cisplatin 50 mg/m 2 plus cyclophosphamide 500 mg/m 2 every 3 weeks for eight cycles or high-dose cisplatin 100 mg/m 2 plus cyclophosphamide 1000 mg/m 2 every 3 weeks for four cycles (same total dose in half the time). In patients with measurable disease, the overall response and clinical complete response rates were equivalent. There was no difference in PFS or OS in the subsets of patients with measurable or nonmeasurable disease. The results of this study did not support the concept that increased dose intensity will produce greater therapeutic effect of cisplatinum or cyclophosphamide. Colombo and associates reported a randomized trial in patients with advanced disease who were given cisplatin 75 mg/m 2 every 3 weeks for six cycles or cisplatin 50 mg/m 2 per week for 9 weeks (same total dose in half the time). Again, no significant differences were seen in clinical or pathologic complete response rates, PFS, or OS. Although neither of these randomized trials of pure dose intensity in cisplatin showed an advantage for a higher dose intensity across a clinically relevant range of doses, other lines of evidence have been cited to support the use of higher dose schedules.
In trials conducted by the National Cancer Institute (NCI) of the United States, even higher dose intensities of cisplatin in conjunction with hypertonic saline to protect the kidneys had been stated to yield higher response rates than more standard doses. A closer examination of these data reported by Rothenberg and coworkers shows confusing results. In patients with large-volume disease, the NCI regimen complete response rates were no different (11% vs. 12%) than the GOG results with CAP, as reported by Omura, regimens with a 3.3-fold difference in dose intensity of cisplatin. In patients with small-volume disease, the NCI regimen complete response rates were not statistically different (38% vs. 30%) from the GOG results with a two-drug combination of cisplatin plus cyclophosphamide, as reported by Omura in 1989. There appears to be no evidence from these data to support the importance of dose intensity of cisplatin over a 3.3-fold range of doses. At least 11 randomized trials have compared standard doses of carboplatin- or cisplatin-based chemotherapy with double doses of the same platinum analogs. Most of these trials showed no advantage in most outcome parameters. A second meta-analysis of the Levin and Hryniuk meta-analysis (in which it appeared that dose intensity had a positive impact on response rate and survival) suggested that platinum dose intensity is unimportant, although intensity of all administered drugs is important, as is tumor residual volume at initiation of therapy.
Katsumata and colleagues from the Japan GOG reported their results of their phase III trial in advanced ovarian cancer evaluating IV carboplatin and paclitaxel dosed every 3 weeks versus IV carboplatin with weekly dose-dense paclitaxel. For each 3-week cycle of therapy, the weekly dose-dense arm received 240 mg/m 2 of paclitaxel versus 180 mg/m 2 of paclitaxel in the control arm. PFS and 3-year OS were significantly improved in the weekly paclitaxel arm (28 vs. 17 months, 72% vs. 65%), with a higher incidence of grade III and IV anemia. Pignata and colleagues reported results from the similar Multicentre Italian Trials in Ovarian Cancer 7 (MITO-7) trial but showed no difference in PFS or OS for weekly versus paclitaxel every 3 weeks. GOG investigators for GOG 262 investigated this question of carboplatin plus weekly paclitaxel versus paclitaxel every 3 weeks in a similar manner to the Japanese Gynecologic Oncology Group (JGOG), with the addition of with or without bevacizumab in each arm. Preliminary results reported no difference in PFS between the arms if bevacizumab was received, but without bevacizumab, the weekly paclitaxel arm had improved PFS.
For 2 decades, high-dose chemotherapy with autologous bone marrow transplantation (ABMT) or peripheral blood stem cell support has been reported for several solid tumors, and there has been a tendency for improved response rates but a relatively short period of disease-free survival in most of the reports reviewed. Many reports show that even in advanced cases, the most important factor for long-term survival after high-dose chemotherapy is the completeness of the primary cytoreductive surgery. Most of the reports in the literature with high-dose chemotherapy with ABMT have been phase II trials of patients with a mixed response to first-line chemotherapy. The best response and survival have been in patients who had had a complete response to pretransplantation chemotherapy. The MD Anderson group reported its experience of 96 patients undergoing transplantation, of whom 43% were in clinical remission. The overall 6-year survival rate was 38% and was 53% in the clinical remission group. Bengala and colleagues reported the results of the European Group for Blood and Marrow Transplantation (EBMT) for 91 patients in first complete remission treated with high-dose chemotherapy and ABMT. The median survival time was 44 months, and they were unable to identify a subgroup more likely to benefit. These reported median survival times are not significantly better than that with use of paclitaxel as second-line therapy. However, the toxicity of high-dose chemotherapy is a serious concern, as is the additional expense. Although interesting, it appears that targeting of patients for high-dose therapy with bone marrow support cannot be considered standard therapy and should only be done as part of an established national protocol so that information can be obtained to increase our knowledge of this therapy.
Immunotherapy
In the past 3 decades, there has been considerable interest in combining chemotherapy with immunotherapy for better results in patients with epithelial cancer of the ovary. The GOG and others have investigated immunomodulating agents such as Corynebacterium parvum and bacille Calmette-Guérin (BCG). Results have been conflicting as to whether nonspecific immunostimulation combined with chemotherapy improves survival.
The potential role of immune response modifiers in the treatment of ovarian cancer has been investigated. Ovarian cancer is a suitable model for biologic therapies because the peritoneal cavity is capable of mounting an inflammatory response to many stimuli, and this response has been shown to induce an antitumor effect. Early experimental observations confirmed that ovarian cancer is a suitable prototype tumor in which to evaluate novel immunotherapeutic and chemoimmunotherapeutic approaches. Berek and coworkers have reported that recombinant interferon alfa has clinical activity in patients with small-volume residual disease. Numerous phase II trials, using substances such as tumor necrosis factor, interleukin-2, and the like, along with other studies using combinations of cytokines have failed to demonstrate significantly improved outcomes. Combining biologic immune response modifiers with standard chemotherapy has proved to be more difficult than initially conceived because of overlapping toxicities. In view of this, it seems prudent to await proven efficacy before attempting to launch the phase III trials with biologic immune response modifiers.
Recent results from trials using programmed cell death protein 1 (PD-1) inhibitors, such as nivolumab and avelumab, have shown some promise. PD-1 is a T-cell “programmed cell death” receptor on T cells that can blocked by a tumor-secreted ligand, inactivating the T cell and allowing the cancer to evade the host immune system. Competitive blockade of this ligand should allow improved cancer immune regulation, and initial results reported by Brahmer and associates in melanoma, non–small cell lung cancer, renal cell cancer, and ovarian cancer were promising. Hamanishi and colleagues have reported their initial results with nivolumab in a cohort of patients with platinum-resistant ovarian cancer, with an encouraging safety profile and activity, including two durable responses. NRG Oncology and others have open trials investigating this mechanism in ovarian cancer. We await the results of these endeavors as to whether these novel treatments will show the same promise in ovarian cancer that has been demonstrated thus far in melanoma.
Intraperitoneal Chemotherapy
Ovarian cancer is predominantly a disease limited to the peritoneal cavity for most patients. For some chemotherapeutic agents, IP administration offers pharmacokinetic advantages, including high IP drug concentration and longer exposure in the peritoneal cavity. Although IP chemotherapy proposals date back decades, it has only been over recent years that the accumulated clinical trial data favor this approach.
Results from randomized trials, listed in Table 11.21 , summarize the experience of clinical trials conducted since the mid-1980s. These studies, all primary therapy trials, compare IV chemotherapy with combined IV and IP chemotherapy. The initial IP study demonstrating that IP chemotherapy may represent a survival advantage was a combined Southwestern Oncology Group (SWOG)/GOG trial (SWOG 8501 and GOG 104). The study compared IV cisplatin and IV cyclophosphamide with IP cisplatin and IV cyclophosphamide in patients with less than 2-cm-diameter residual disease. The IP arm produced a median survival of 49 months compared with 41 months for the IV arm, with an HR of 0.77. However, critics pointed out that the subsequent introduction of paclitaxel to our frontline treatment may have neutralized this apparent advantage for IP therapy. The other concern is that accrual was extended beyond the initial study design for patients with 0- to 0.5-cm residual disease, and still this subgroup failed to demonstrate superior survival (51 months vs. 46 months; HR, 0.80). One would have expected the treatment difference to be most pronounced in this patient subgroup. Partly because of these issues, IP therapy was not uniformly supported as producing a survival advantage over the current IV standard, carboplatin-paclitaxel.
| Study Identifier (Author, Year Published) | Control Regimen | Experimental Regimen | Target Population | Patients ( n ) | Median Duration of Survival for Control Regimen (Months) | Median Duration of Survival for Experimental Regimen (Months) |
|---|---|---|---|---|---|---|
| SWOG/GOG-104 ( ) | Cisplatin 100 mg/m 2 IV, ctx 600 mg/m 2 IV q 3 weeks × 6 | Cisplatin 100 mg/ m 2 IP, ctx 600 mg/m 2 IV q 3 weeks × 6 | Stage III ≤2 cm residual | 546 | 41 | 49 |
| Greek ( ) | Carboplatin 350 mg/m 2 IV, ctx 600 mg/m 2 IV q 3 weeks × 6 | Carboplatin 350 mg/m 2 IV, ctx 600 mg/m 2 IV q 3 weeks × 6 | Stage III ≤or >2 cm residual | 90 | 52 | 63 |
| GONO ( ) | Cisplatin 50 mg/m 2 IV, ctx 600 mg/m 2 IV, epidox 60 mg/m 2 IV q 4 weeks × 6 | Cisplatin 50 mg/m 2 IP, ctx 600 mg/m 2 IV, epidox 60 mg/m 2 IV q 4 weeks × 6 | Stage II or IV <2 cm residual | 113 | 25 | 26 |
| GOG-114/SWOG ( ) | Cisplatin 75 mg/m 2 IV, tax 135 mg/m 2 (24 hr) IV q 3 weeks × 6 | Carboplatin AUC 9 IV q 28 days × 2, cisplatin 100 mg/m 2 IP, tax 135 mg/m 2 (24 hr) IV q 3 weeks × 6 | Stage III ≤1 cm residual | 462 | 51 | 67 |
| Taiwan ( ) | Cisplatin 50 mg/m 2 IV, ctx 500 mg/m 2 IV epi/adr 50 mg/m 2 IV q 3 weeks × 6 | Cisplatin 100 mg/m 2 IV, ctx 500 mg/m 2 IV, epi/adr 50 mg/m 2 IV q 3 weeks × 6 | Stage III ≤1 cm residual | 118 | 48 | 43 |
| GOG-172 (Armstrong et al., 2006) | Cisplatin 75 mg/m 2 IV, tax 135 mg/m 2 (24 hr) IV q 3 weeks × 6 | Tax 135 mg/m 2 (24 hr) IV, cisplatin 100 mg/m 2 IP, tax 60 mg/m 2 IV on day 8q 3 weeks × 6 | Stage III ≤1 cm | 415 | 49 | 67 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree