Aza C
Supportive care
Crossover
No. of patients
%
No. of patients
%
No. of patients
%
No. evaluated
99
92
49
CR
7
7*
0
0
5
10
PR
16
16*
0
0
2
4
Improved
37
37*
5
5
16
33
Total
60
60*
5
5
23
47
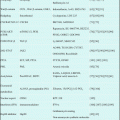
In response to the results of CALGB9221 study, a multicenter, international, randomized phase III study (AZA-001) was designed to compare the overall survival benefit of AZA treatment with that of the conventional care regimens (CCR) for high-risk MDS [23]. In this study, BSC, low-dose cytarabine (Ara-C) (Ara-C: 20 mg/m2 s.c. for 14 days), and intensive chemotherapy (ICT) (Ara-C: 100–200 mg/m2 + daunorubicin: 45–60 mg/m2 for 3 days or idarubicin: 9–12 mg/m2 for 3 days or mitoxantrone: 8–12 mg/m2 for 3 days) were included in CCR arm. Before randomization, the patient and doctor were asked to select one treatment from CCR to be administered if assigned to the CCR arm, and then a randomization was done between AZA and the CCR treatment selected. This trial enrolled 358 patients with high-risk MDS, assessed by an independent review committee for FAB subtypes and International Prognostic Scoring System (IPSS) risk category [24]. One hundred seventy-nine patients were randomized to AZA arm (75 mg/m2 s.c. for 7 days in a 28-day cycle) and 179 patients were randomized to CCR arm. The patients allocated for CCR arm received either BCS alone (n = 105), low-dose Ara-C (n = 49), or ICT (n = 25). Median age of patients was 69 years old. Median overall survival was 24.5 months for patients in the AZA arm compared with 15 months in CCR arm, and a statistically significant difference was observed (P = 0.0001). Time to progression to AML was also significantly prolonged in AZA arm. The survival benefit with AZA was unconcerned with age, percentage of marrow blasts, or chromosomal karyotype (Table 19.2). This study for the first time demonstrated that AZA prolonged overall survival of high-risk MDS patients over other available treatment regimens at that time. By now, AZA is the only one agent to be proven for survival benefit other than allogeneic transplantation. Since allogeneic hematopoietic stem cell transplantation is not indicated for most of MSD patients with various reasons, such as high age, comorbidities, etc., AZA is the first choice for high-risk MDS that lack the indication of transplant.
BSC only (n = 222) | Low-dose cytarabine (n = 94) | Intensive chemotherapy (n = 42) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Azacitidine (n = 117) | BSC (n = 105) | HR (95% CI) | p value | Azacitidine (n = 45) | Low-dose cytarabine (n = 49) | HR (95% CI) | p value | Azacitidine (n = 17) | Intensive chemotherapy (n = 25) | HR (95% CI) | p value | |
Overall survival (months) | 21.1 (10.5-NR) | 11.5 (5.7-NR) | 0.58 (0.40–0.85) | 0.0045 | 24.5 (8.4–34.7) | 15.3 (4.9–25.8) | 0.36 (0.20–0.65) | 0.0006 | 25.1 (10.0-NR) | 15.7 (8.2–24.1) | 0.76 (0.33–1.74) | 0.51 |
Time to transformationto AML (months) | 15.0 (8.8–27.6) | 10.1(3.9–19.8) | 0.41 (0.27–0.63) | <0.0001 | 15.0 (7.3–25.5) | 14.5 (4.9–19.2) | 0.55 (0.28–1.11) | 0.097 | 23.1 (6.4–25.4) | 10.7 (4.6–15.4) | 0.48 (0.16–1.45) | 0.19 |
Additional analyses of AZA-001 trial revealed interesting features of AZA in the treatment of high-risk MDS. In the induction therapy for AML, hematologists expect to achieve CR after one course of treatment, and patients that entered CR after one course of induction regimen usually show better survival (or longer CR duration) than those obtained CR after two or more courses of induction therapy, showing the importance of prompt reduction of leukemia cells by chemotherapy. However, in terms of AZA for MDS, almost 40% of responders show HI or other responses after four courses of AZA treatment (usually more than 4 months after the initial injection). Time to response did not have strong impact on survival as for MDS as AML. Interestingly, survival benefit among patients with stable disease was also suggested after AZA treatment. Not only pharmacologically but also clinically, AZA shows different features than classical chemotherapeutic agents including Ara-C. Considering no other standard therapy is available for high-risk MDS, it makes physicians continue AZA treatment until progression or intolerable situation.
19.2.2 Clinical Studies of Decitabine in MDS
Decitabine (DAC) is another nucleoside analog with the capacity to induce DNA hypomethylation through the inhibition of DNMT. The mechanism of hypomethylation of DNA is the same as seen in AZA. Since AZA but no other treatment including chemotherapy with anthracyclins and Ara-C showed clinical efficacy for MDS, DNA hypomethylation was thought to be very important for the treatment of MDS. Considering the mode of action of DAC in which 100% of DAC theoretically works as DNMT inhibitor, DAC was expected to show higher efficacy than AZA of which about 20% would be incorporated into DNA to inhibit DNMT. The clinical trials with the middle or the high dose of DAC were conducted initially. However, there was no preeminence with many adverse events as compared with other drug, and then clinic development was discontinued. After, the demethylation activity of DAC drew attention, and clinical trials with low-dose DAC were conducted. Wijermans et al. conducted a phase II clinical study with decitabine for 66 patients of MDS to test the efficacy and toxicity of DAC [25]. DAC was administered at a dose of 15 mg/m2 infusion over a 4 h period every 8 h (45 mg/m2/day) for 3 consecutive days. DAC treatment courses were repeated every 6 weeks up to six courses. Primary endpoint was response rate. There were eight cases of RA or RARS, 29 of RAEB, 20 of RAEB-T, and nine CMML cases, and most of them (76%, 50 out of 66 cases) were included in the higher-risk group of IPSS category. Among them, 32 patients (49%) showed clinical response, including CR (n = 13, 19.7%), PR (n = 3, 4.5%), and improvement (n = 16, 24.2%) of cytopenia as defined by the protocol (reduction of at least 50% of transfusion requirements, etc.). Response rates by the risk category of IPSS were as follows: 25% in the intermediate (Int)-1 category, 48% in Int-2, and 64% in high-risk category. The median time of response duration was 31 weeks. These suggested the clinical efficacy of DAC over the risk of MDS predicted by IPSS. The treatment-related mortality (toxic death) was 7.6% (n = 5), and non-hematological toxicity of grade 3–4 was observed at only 13.6% (n = 9). There were 17 cases (26%) of disease progression. These results were comparable or even better than those of AZA trials mainly conducted by CALGB (mentioned above).
Based on these, a randomized trial was performed in which response rate and time to AML or death were compared between DAC with BSC and BSC alone in MDS at 18 years or older [26]. In this study, the dose of decitabine was 15 mg/m2 and administered intravenously over 3 h every 8 h for 3 days (at a dose of 45 mg/m2/day and 135 mg/m2 per course) and which repeated every 6 weeks depending on the recovery from myelosuppression, almost the same schedule as in the previous phase II study. Response was assessed according to the International IWG criteria [19]. A total of 170 patients with MDS (median age was 70 years old) were randomized to receive either DAC or BSC. Fifty-two patients (31%) were categorized into Int-1 risk group of IPSS, 74 patients (44%) into Int-2 risk group of IPSS, and 43 (25%) in high-risk group of IPSS. Overall response rate was 17% (9% with CR and 8% with PR) in DAC and 0% in BSC, and clinical improvement that included CR, PR, and HI was obtained in 30% of cases in DAC arm and 7% in BSC arm. There were statistically significant differences both in overall response rate and clinical improvement rate (both P < 0.001). Although DAC group showed a trend toward a longer median time to AML or death than BSC group, there was no significant difference between the two groups (12.1 months for DAC and 7.8 months for BSC, respectively, P = 0.16) (Table 19.3). These results led to the approval of DAC for MDS in the USA.
Decitabine (n = 89) (%) | Supportive care (n = 81) (%) | P value* | |
|---|---|---|---|
Clinical response | |||
Overall response (CR + PR) | 15(17) | 0 | <0.001 |
CR | 8(9) | 0 | |
PR | 7(8) | 0 | |
Clinical improvement | |||
Overall improvement (CR + PR + HI) | 27(30) | 6(7) | <0.001 |
HI | 12(13) | 6(7) | |
Major | 12(13)a | 5(6)b | |
Minor | 0(0) | 1(1) | |
Response by subgroup (CR + PR) | |||
A similar phase III clinical study of DAC for MDS was conducted among European countries by European Organization for Research and Treatment of Cancer Leukemia Group (EORTC) [27]. The administration method of DAC was similar to the prior phase III study, and the primary endpoint was set as overall survival (OS). Two-hundred thirty-three patients were enrolled; 92% of patients had high-risk diseases (IPSS Int-2 and high) with 32% of AML by WHO criteria, and 53% had poor-risk cytogenetics. These patients were randomly assigned to decitabine arm (n = 119) and the BSC arm (n = 114), with a balanced distribution for age, sex, performance status, and risk profile (IPSS subgroup and FAB subtype). DAC (15 mg/m2) was given intravenously over 4 h three times a day for 3 days in 6-week cycles. The maximum number of treatment cycle was limited to eight (or ten in case CR was achieved after eight courses). As the primary endpoint, OS prolongation in DAC arm was not shown over BSC with statistical significance (median OS, 10.1, and 8.5 months, respectively, P = 0.38). Progression-free survival (PFS), defined as time from randomization to progression, relapse after CR or PR or death, prolonged significantly by DAC (1-year PFS rate 27% and 15%, in the DAC and BSC arm, respectively, P = 0.004). There was no statistical difference in AML-free survival (AMLFS, defined as time from randomization to AML transformation or death) between the DAC and BSC arms (median AMLFS, 8.8 versus 6.1 months, respectively, P = 0.24), although AML transformation was significantly (P = 0.036) reduced at 1 year (from 33% with BSC to 22% with DAC). There was no difference in cumulative incidence of death without AML, either between two arms (P = 0.17). Additional analyses suggested the advantage of DAC over BSC in PFS among several subgroups. In terms of toxicity, grade 3 or 4 infections were most frequent AEs followed by gastrointestinal events. Neutropenia and fatigue were also frequently observed, as seen in other DAC studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree