Overexpression of epidermal growth factor receptor (EGFR) is linked with poor prognosis in squamous cell carcinoma of the head and neck (SCCHN). Cetuximab binds specifically to EGFR with high affinity; combined with radiotherapy, it improves locoregional control and survival over radiotherapy alone. Adding cetuximab to platinum-based chemotherapy and 5-fluorouracil improves overall survival in incurable disease. Only a minority of patients benefit from anti-EGFR monoclonal antibodies. A better understanding of the molecular mechanisms involved in treatment resistance and identification of predictive biomarkers are crucial. Potentially more potent anti-EGFR compounds are currently under investigation with the aim of improving treatment efficacy.
Key points
- •
Up to 90% of squamous cell carcinomas of the head and neck express high levels of epidermal growth factor receptor (EGFR).
- •
Overexpression and high EGFR gene copy number are associated with poor prognosis.
- •
Cetuximab improves overall survival either as curative treatment in combination with radiation therapy or as palliative treatment in combination with chemotherapy.
- •
A minority of patients derive long-term benefit of anti-EGFR treatment, outlining the importance of developing novel treatment strategies.
- •
Potentially more potent anti-EGFR compounds as well as combination strategies are under investigation to improve treatment efficacy.
The epidermal growth factor receptor
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein commonly expressed in many normal tissues. It is a member of the human epidermal receptor (HER) tyrosine kinase receptor family composed of 4 different receptors (EGFR/c-erbB-1, c-erbB-2/HER-2/neu, c-erbB-3/HER-3 and c-erbB4/HER-4), all of which are transmembrane proteins with tyrosine kinase activity ( Fig. 1 ).
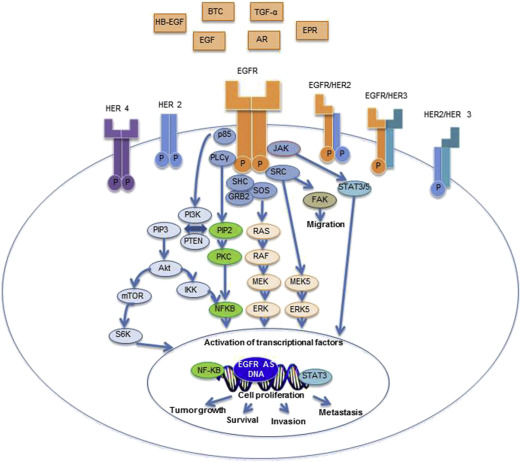
The EGFR has an extracellular domain, which provides a ligand-binding site for multiple ligands. Upon ligand fixation, EGFR homodimerization or heterodimerization with another HER receptor occurs, leading to the activation of the intracellular tyrosine kinase. This leads to activation of molecular pathways involved in tumor proliferation, apoptosis, angiogenesis, and cell migration/invasion. Downstream signaling through the Ras/Raf/Mek/Erk pathway controls gene transcription, cell proliferation, and cell-cycle progression, whereas the phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) pathway stimulates numerous antiapoptotic signals. Other proteins activated by EGFR include the Src tyrosine kinase, the phospholipase-C gamma, the protein kinase C, and the signal transducer and activator of transcription.
Up to 90% of squamous cell carcinoma of the head and neck (SCCHN) express high levels of EGFR and overexpression of EGFR and transforming growth factor-α are associated with poor prognosis and radioresistance. Increased EGFR gene copy number has been reported in 10% to 58% of patients with SCCHN and has also been described as an indicator of poor survival, locoregional failure, and radioresistance.
In non–small cell lung cancer, EGFR mutations (especially in exons 19 and 20) are correlated with better treatment response to EGFR tyrosine kinase inhibitors. These mutations are, however, rarely found in SCCHN. EGFRvIII is the result of an in-frame deletion of exons 2 through 7 (deletion of amino acids 30–297, involving 801 base pairs), resulting in a truncated extracellular EGF-binding domain that is constitutively activated. Although earlier reports documented EGFRvIII in about 40% of SCCHN, EGFRvIII variant was identified in less than 0.5% of the samples analyzed by the Cancer Genome Atlas project. The reasons for this discrepancy are unknown and require further investigation (methodologic issues, difference between early stage and recurrent SCCHN, etc).
The epidermal growth factor receptor
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein commonly expressed in many normal tissues. It is a member of the human epidermal receptor (HER) tyrosine kinase receptor family composed of 4 different receptors (EGFR/c-erbB-1, c-erbB-2/HER-2/neu, c-erbB-3/HER-3 and c-erbB4/HER-4), all of which are transmembrane proteins with tyrosine kinase activity ( Fig. 1 ).
The EGFR has an extracellular domain, which provides a ligand-binding site for multiple ligands. Upon ligand fixation, EGFR homodimerization or heterodimerization with another HER receptor occurs, leading to the activation of the intracellular tyrosine kinase. This leads to activation of molecular pathways involved in tumor proliferation, apoptosis, angiogenesis, and cell migration/invasion. Downstream signaling through the Ras/Raf/Mek/Erk pathway controls gene transcription, cell proliferation, and cell-cycle progression, whereas the phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) pathway stimulates numerous antiapoptotic signals. Other proteins activated by EGFR include the Src tyrosine kinase, the phospholipase-C gamma, the protein kinase C, and the signal transducer and activator of transcription.
Up to 90% of squamous cell carcinoma of the head and neck (SCCHN) express high levels of EGFR and overexpression of EGFR and transforming growth factor-α are associated with poor prognosis and radioresistance. Increased EGFR gene copy number has been reported in 10% to 58% of patients with SCCHN and has also been described as an indicator of poor survival, locoregional failure, and radioresistance.
In non–small cell lung cancer, EGFR mutations (especially in exons 19 and 20) are correlated with better treatment response to EGFR tyrosine kinase inhibitors. These mutations are, however, rarely found in SCCHN. EGFRvIII is the result of an in-frame deletion of exons 2 through 7 (deletion of amino acids 30–297, involving 801 base pairs), resulting in a truncated extracellular EGF-binding domain that is constitutively activated. Although earlier reports documented EGFRvIII in about 40% of SCCHN, EGFRvIII variant was identified in less than 0.5% of the samples analyzed by the Cancer Genome Atlas project. The reasons for this discrepancy are unknown and require further investigation (methodologic issues, difference between early stage and recurrent SCCHN, etc).
Inhibition of epidermal growth factor receptor
There are 2 ways to inhibit EGFR signaling ( Fig. 2 ). First, monoclonal antibodies (mAbs) administered intravenously can bind specifically to the EGFR with high affinity and thereby block ligand-binding–induced receptor activation. Second, oral tyrosine kinase competitive inhibitors can reversibly or irreversibly inhibit the binding of adenosine 5’-triphosphate to the phosphate-binding loop of the adenosine 5’-triphosphate binding site in the intracellular domain of EGFR, thereby abrogate downstream signaling.
Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies
In SCCHN, cetuximab, panitumumab, and zalutumumab are the most investigated mAbs that specifically bind to EGFR with high affinity. Cetuximab is a chimeric human/murine immunoglobulin (Ig)G1 mAb, whereas zalutumumab (IgG1) and panitumumab (IgG2) are fully human mAbs. In addition to this direct effect on the EGFR, cetuximab and zalutumumab could also activate antibody-dependent cellular cytotoxicity through the binding of their Fc tail to Fc gamma receptors on natural killer cells, monocytes, dendritic cells, and other granulocytes. EGFR blockade is also thought to possibly act via inhibition of DNA double-strand break repair that contributes to resistance to radiotherapy and DNA damage induced by chemotherapeutic agents by preventing nuclear import of EGFR. The main trials investigating anti-EGFR mAbs in SCCHN are summarized in Table 1 .
| Trial | Regimens | N | Disease Stage | Locoregional Control | Progression-Free Survival | Overall Survival |
|---|---|---|---|---|---|---|
| Radiotherapy vs radiotherapy plus an anti-EGFR mAb | ||||||
| Bonner (phase III) | Radiotherapy + cetuximab versus | 213 | Stage III or IV squamous cell carcinoma of the head and neck | 24.4 mo a (median) | 17.1 mo a (median) | 49 mo a (median) |
| Radiotherapy | 211 | 14.9 mo (median) | 12.4 mo (median) | 29 mo (median) | ||
| Chemoradiation vs radiotherapy plus an anti-EGFR mAb | ||||||
| GSTTC Italian study group (phase III) | Radiotherapy + cetuximab versus | 161 | Stage III or IV squamous cell carcinoma of the head and neck | NA | 20.7 mo (median) | 44.7 mo (median) |
| Chemoradiation (2 cycles of cisplatin/5-fluorouracil) | 260 | 21.6 mo (median) | 44.7 mo (median) | |||
| CONCERT-2 (phase II) | Radiotherapy + panitumumab versus | 90 | Stage III or IV squamous cell carcinoma of the head and neck | 61% (rate at 2 y) | 41% (rate at 2 y) | 63% (rate at 2 y) |
| Chemoradiation (2 cycles of high-dose cisplatin) | 61 | 51% (rate at 2 y) | 62% (rate at 2 y) | 71% (rate at 2 y) | ||
| NCIC clinical trial group HN.6 trial (phase III) | Radiation therapy (70 Gy) + cisplatin 100 mg/m 2 d 1, 22, 43 versus | 156 | Stage III or IV squamous cell carcinoma of the head and neck | — | 73% (rate at 2 y) | 85% (rate at 2 y) |
| Accelerated radiotherapy + panitumumab day -7, 15, 36 | 159 | 76% (rate at 2 y) | 88% (rate at 2 y) | |||
| Induction chemotherapy followed by radiotherapy plus an anti-EGFR mAb | ||||||
| TREMPLIN (phase II) | TPF followed by chemoradiation (3 cycles of high-dose cisplatin) versus | 60 | Resectable squamous cell carcinoma of the larynx and hypopharynx | NA | NA | 75% (rate at 3 y) |
| TPF followed by cetuximab plus radiation | 56 | 73% (rate at 3 y) | ||||
| Chemoradiation vs chemoradiation plus an anti-EGFR mAb | ||||||
| RTOG 0522 (phase III) | Chemoradiation (2 cycles of high-dose cisplatin) versus | 447 | Stage III or IV squamous cell carcinoma of the head and neck | NA | 61% (rate at 3 y) | 73% (rate at 3 y) |
| Chemoradiation plus cetuximab | 444 | 59% (rate at 3 y) | 76% (rate at 3 y) | |||
| CONCERT-1 (phase II) | Chemoradiation (3 cycles of high-dose cisplatin) versus | 63 | Stage III or IV squamous cell carcinoma of the head and neck | 68% (rate at 2 y) | 65% (rate at 2 y) | 78% (rate at 2 y) |
| Chemoradiation plus panitumumab | 87 | 61% (rate at 2 y) | 61% (rate at 2 y) | 69% (rate at 2 y) | ||
| DAHANCA 19 (phase III) | (Chemo)radiation plus nimorazole versus | 309 | Squamous cell carcinoma of the head and neck (89% were stage III and IV; 70% received chemoradiation) | 73% (rate at 4 y) | NA | Hazard ratio: 1.22 |
| (Chemo)radiation plus nimorazole and zalutumumab | 310 | 71% (rate at 4 y) | ||||
| Anti-EGFR mAbs as adjuvant therapy | ||||||
| Mesia (phase II) | Radiotherapy plus concomitant cetuximab versus | 46 | Stage III/IV oropharyngeal cancer | 44% (rate at 2 y) | 35.3 mo (median) | 33.6 mo (median) |
| Radiotherapy plus concomitant and adjuvant cetuximab (12 wk) | 45 | 44% (rate at 2 y) | 41 mo (median) | 39.9 mo (median) | ||
| Anti-EGFR mAbs in recurrent and/or metastatic (R/M) disease | ||||||
| ECOG E5397 (phase III) | Cisplatin versus | 60 | R/M squamous cell carcinoma: first-line | NA | 2.7 mo (median) | 8 mo (median) |
| Cisplatin and cetuximab | 57 | 4.2 mo (median) | 9.2 mo (median) | |||
| EXTREME (phase III) | Platin/5-fluorouracil versus | 220 | R/M squamous cell carcinoma: first-line | NA | 3.3 mo a (median) | 7.4 mo a (median) |
| Cisplatin/5-fluorouracil/cetuximab | 222 | 5.6 mo a (median) | 10.1 mo a (median) | |||
| SPECTRUM (phase III) | Cisplatin/5-fluorouracil versus | 330 | R/M squamous cell carcinoma: first-line | NA | 4.6 mo a (median) | 9 mo (median) |
| Cisplatin/5-fluorouracil/panitumumab | 327 | 5.8 mo a (median) | 11.1 mo (median) | |||
| PARTNER (randomized phase II) | Cisplatin/docetaxel versus | 51 | R/M squamous cell carcinoma: first-line | NA | 5.5 mo (median) | 13.8 mo (median) |
| Cisplatin/docetaxel/panitumumab | 52 | 6.9 mo (median) | 12.9 mo (median) | |||
| ZALUTE (phase III) | Best supportive care or methotrexate versus | 95 | R/M squamous cell carcinoma: second-line (platinum failure) | NA | 8.4 wk a (median) | 5.2 mo (median) |
| Zalutumumab | 191 | 9.9 wk a (median) | 6.7 mo (median) | |||
The role of anti-epidermal growth factor receptor monoclonal antibodies in the multimodal curative treatment
Radiotherapy plus anti-epidermal growth factor receptor monoclonal antibodies versus radiotherapy
Bonner and colleagues compared radiotherapy alone with radiotherapy plus cetuximab in stage III/IV SCCHN. They found that the addition of cetuximab improved median overall survival (OS) from 29.3 to 49.0 months ( P = .03) and locoregional control (LCR) from 14.9 to 24.4 months ( P = .005). Because the patients with oropharynx cancer seemed to be the subgroup that benefitted the most from cetuximab, the human papilloma virus (HPV) status using tumor p16 as a surrogate marker was assessed retrospectively. The univariate analysis showed a more pronounced treatment effect of radiotherapy and cetuximab versus radiotherapy in patients with p16-positive tumors. However, interaction tests in p16-positive and -negative populations did not demonstrate a significant interaction between p16 status and treatment effect. Therefore, we cannot conclude definitively from this trial that p16 positivity is a predictive biomarker for cetuximab activity in this setting.
Radiotherapy plus anti-epidermal growth factor receptor monoclonal antibodies versus chemoradiation
The GSTTC Italian Study Group randomized patients with unresectable stage III/IV SCCHN according to a 2 × 2 factorial design: 2 cycles of cisplatin/5-fluorouracil concomitant to radiotherapy (arm A1), cetuximab concomitant to radiotherapy (arm A2), 3 cycles of induction cisplatin, docetaxel, and 5-fluorouracil (TPF) followed by concurrent platinum-based chemoradiation (arm B1) and 3 cycles of TPF followed by cetuximab and radiotherapy (arm B2). Interestingly, no differences for grades 3 and 4 in-field skin and mucositis toxicities were observed, challenging the concept that cetuximab added to radiation therapy is less toxic than cisplatin-based chemoradiation. Both regimens showed similar efficacy: median progression-free survival (PFS) and OS were 21.6 months and 44.7 months for the chemoradiation arm and 20.7 months and 44.7 months for the cetuximab and radiotherapy arm. However, this study was not adequately powered to demonstrate that cetuximab plus radiation therapy is equivalent to cisplatin-based chemoradiation.
In the CONCERT-2 trial, the investigators compared the safety and efficacy of radiation therapy combined with panitumumab versus concurrent chemoradiation. The investigators reported a trend in favor of concurrent chemoradiation for LCR at 2 years (the primary endpoint): 51% with irradiation plus panitumumab compared with 61% with concurrent chemoradiation. Both PFS ( P = .03) and OS ( P = .10) outcomes favored the concurrent chemoradiation arm.
The NCI Canadian group randomized 320 stage 3 and 4 SCCHN patients between standard fractionated radiotherapy (70 Gy over 7 weeks) plus cisplatin (100 mg/m 2 , 3 cycles) versus accelerated radiotherapy (70 Gy over 6 weeks) plus panitumumab (9 mg/kg for 3 doses). The 2-year PFS was 73% and 76%, respectively. In addition, noninferiority of bioradiotherapy was not proven in this study.
Therefore, so far, in locally advanced (LA) SCCHN, the combination of radiation therapy and anti-EGFR mAb has not been proven to be equivalent to platinum-based chemoradiation. The results of the RTOG 1016 study that randomized p16-positive oropharyngeal cancer patients between accelerated chemoradiation (high-dose cisplatin) and accelerated radiotherapy plus cetuximab are pending. Outside clinical trials, platinum-based chemoradiation remains the standard of care for fit patients. Cetuximab plus radiotherapy should be only considered for patients with stage 3 and 4 SCCHN and when contraindications to cisplatin such as kidney dysfunction, hearing loss, or cardiovascular insufficiency are present.
Anti-epidermal growth factor receptor monoclonal antibodies and concurrent radiotherapy after induction chemotherapy
Lefebvre and colleagues reported the results of a phase II trial comparing the efficacy and safety of induction chemotherapy (TPF) followed by concurrent regimens for larynx preservation (TREMPLIN). Poor responders to induction chemotherapy (<50% response) underwent salvage surgery. Responders were assigned randomly to conventional radiotherapy plus either cisplatin or cetuximab. The primary end point was 3-month larynx preservation. In an intent-to-treat analysis, there was no difference between the arms in the rates of larynx preservation at 3 months and OS at 18 months, but this study was not powered adequately to address these questions. Treatment compliance was higher in the cetuximab plus radiation therapy arm.
In the GSTTC Italian Study Group described, an analysis that compared the induction versus noninduction arms was reported recently. The authors found that induction TPF followed by chemoradiation or concurrent cetuximab plus radiotherapy significantly improved PFS and OS (53 vs 30 months ( P = .015); independent from the type of concomitant strategy). The benefit of induction chemotherapy seemed to be greater in the cetuximab arm and for patients with non-oropharyngeal cancer. However, the 2 × 2 factorial design decreases the number of patients in each arm, precluding definitive conclusions owing to a lack of statistical power. With the data we have available presently, TPF followed by concurrent cetuximab and radiotherapy cannot be considered as a standard of care.
Chemoradiation versus chemoradiation plus anti-epidermal growth factor receptor monoclonal antibodies
The RTOG 0522 trial investigated the addition of cetuximab to platinum-based chemoradiation in patients with stage III/IV SCCHN. No difference was observed between the 2 groups. The 3-year PFS in the chemoradiation group was 61%, whereas in the chemoradiation group plus cetuximab it was 59%. The 3-year OS was 73% and 76%, respectively. However, local toxicity was higher in the cetuximab-containing arm, with grade 3 and 4 stomatitis being observed in 43% versus 33% and in-field dermatitis in 25% versus 15%.
The phase II CONCERT-1 study showed that the addition of panitumumab to cisplatin-based chemoradiation resulted in increased toxicity with no improvement in response rate or survival. LCR at 2 years was 68% in the chemoradiation group and 61% in the panitumumab group.
The DAHANCA 19 trial randomized 619 patients between accelerated radiotherapy plus nimorazole and, in case of stage 3 and 4 disease, weekly cisplatin versus the same regimens plus zalutumumab. Seventy percent of the patients received cisplatin. The 4-year LCR rate was 71% in the zalutumumab arm and 73% in the control arm. Survival was similar between the 2 arms.
A randomized, phase II trial (RTOG-0234) investigated concurrent chemoradiotherapy and cetuximab in the postoperative treatment of patients with SCCHN with high-risk pathologic features. Patients were randomly assigned to 60 Gy radiation with cetuximab once per week plus either cisplatin 30 mg/m 2 or docetaxel 15 mg/m 2 once per week. The 2-year OS was 69% for the cisplatin arm and 79% for the docetaxel arm; the 2-year disease-free survival was 57% and 66%, respectively. Although these results look promising, they need to be validated or not in a larger trial (RTOG 1216).
Consequently, chemoradiation without anti-EGFR mAb remains the standard of care in LA SCCHN. Most of these trials adding an anti-EGFR mAb to standard (chemo)radiation have been conducted before HPV-induced oropharyngeal cancer was recognized as a different molecular and clinical disease compared with tobacco and alcohol-induced SCCHN. Therefore, it is possible that we have diluted the effect of anti-EGFR mAb in 1 of these subgroups.
Anti-epidermal growth factor receptor monoclonal antibodies as adjuvant therapy
Adjuvant therapy consists of starting or continuing a targeted agent after definitive treatment. A randomized, phase II trial investigated the efficacy and safety of cetuximab maintenance therapy given for 12 weeks after radiotherapy in patients with stage III/IV oropharyngeal cancer. LCR at 1 year was superior among patients treated with cetuximab maintenance (59% vs 47%). However, LCR was similar between both arms after 2 years of follow-up. In another phase II trial, 2-year PFS was 70% in 39 stage III/IVA-B patients who received cisplatin/docetaxel/cetuximab as induction therapy followed by cisplatin/cetuximab/radiotherapy and who then continued cetuximab for a maximum of 6 months. Further trials are needed.
The role of anti-epidermal growth factor receptor monoclonal antibodies in recurrent and/or metastatic disease
First-line treatment in combination with platinum-based chemotherapy
Burtness and colleagues conducted a randomized trial (ECOG E5397) comparing cisplatin and placebo with cisplatin and cetuximab in chemotherapy-naïve SCCHN patients with incurable SCCHN. There was a statistically significant higher response rate in the cetuximab arm, 26% versus 10% ( P = .03), but no difference in PFS (the primary endpoint) or OS.
The phase III EXTREME trial randomized 442 patients with recurrent or metastatic SCCHN to receive 5-fluorouracil and platinum-based therapy alone or in combination with cetuximab as a first-line palliative regimen. In the experimental arm, patients who had at least stable disease after a maximum of 6 cycles of chemotherapy received cetuximab monotherapy until the disease progressed or occurrence of unacceptable toxic effects. This study demonstrated a benefit of cetuximab with an improvement in median OS from 7.4 to 10.1 months ( P = .04). An unplanned retrospective analysis assessed the outcome by tumor p16 status (as a surrogate marker for HPV) and concluded that the survival benefit of adding cetuximab to chemotherapy was independent of tumor p16 status, and that patients with p16-positive tumors had a more favorable outcome than those with p16-negative tumors. However, this last analysis is limited by the low number of patients with p16-positive disease.
The SPECTRUM trial randomized 657 patients with recurrent and/or metastatic SCCHN to cisplatin and 5-fluorouracil alone or with panitumumab, every 3 weeks. Panitumumab did not significantly improve the primary endpoint of OS (median, 11.1 vs 9.0 months; P = .14), but did yield significantly higher objective response rate (ORR; 36% vs 25%; P = .007) and PFS (5.8 vs 4.6 months; P = .004). An analysis of the results stratified by tumor p16 status suggested that panitumumab improved OS and PFS in patients with p16-negative tumors but not in those with p16-positive disease. These results should be interpreted with caution because of the low number of patients with p16-positive tumors as well as the definition of p16 positivity (>10% of the tumor cells expressing p16 in contrast with the more commonly accepted criterion of >70%). In addition, as mentioned, a similar analysis in the EXTREME study, using the 70% cutoff point, did not show the same results. Therefore, further evaluation is needed to better understand the impact of HPV and/or p16 status on the activity of anti-EGFR mAbs.
A randomized (1:1) phase II study investigated docetaxel and cisplatin with or without panitumumab in the first-line palliative treatment. Median PFS, median OS, and overall response rate were 6.9 months, 12.9 months, and 44% in the panitumumab arm versus 5.5 months, 13.8 months, and 37% in the arm without panitumumab, respectively. Crossover to panitumumab monotherapy was allowed in the docetaxel/cisplatin arm and occurred in 57% of the patients, which may have impaired the OS analysis.
Second-line treatment after platinum-based chemotherapy
The results of 2 phase II trials investigating the addition of cetuximab to carboplatin or cisplatin in patients with platinum-refractory SCCHN showed an ORR of 10% and a median survival of 5.2 to 6.1 months. Another phase II study with similar inclusion criteria evaluated cetuximab as monotherapy and reported an ORR of 13% with a median OS of 5.9 months. This last study suggests that single agent cetuximab, in this platinum-refractory population, can offer similar results to those obtained when the drug is used in combination with a platinum compound. A pooled analysis of these three phase II trials (n = 278) was performed and the results were compared with those from Leon’s retrospective study in which patients (n = 151) were treated with best supportive care or various second-line treatments. This indirect comparison suggests that cetuximab has the potential to increase median OS by approximately 2 months. Although a randomized trial is missing in the recurrent, metastatic or palliative setting, cetuximab was approved by the US Food and Drug Administration as monotherapy in recurrent and/or metastatic disease after platinum-based treatment ( www.fda.gov ).
The ZALUTE trial randomized 286 patients with recurrent and/or metastatic SCCHN that progressed within 6 months of platinum-based therapy to receive either zalutumumab plus best supportive care with optional methotrexate in a 2:1 ratio. This study did not meet its primary endpoint of improving OS (median of 6.7 months in the zalutumumab group vs 5.2 months in the control group; P = .06). However, PFS was significantly higher in the zalutumumab group ( P = .001), suggesting clinical activity of this agent.
Cetuximab therapy in the recurrent and/or metastatic setting is considered standard of care by many clinicians. In Europe, cetuximab is mainly used in first-line in combination with platinum-based chemotherapy and in United States it is frequently used in monotherapy, as second-line treatment after platinum failure. However, the cost of anti-EGFR mAbs should be balanced with the limited benefit of this compound. Therefore, some countries do not provide health insurance coverage for cetuximab in noncurable SCCHN.
Other anti-epidermal growth factor receptor monoclonal antibodies with preliminary results
Nimotuzumab (h-R3mAb) is another mAb that bivalently binds to the extracellular domain of the EGFR that overlaps with the binding site of cetuximab and EGF. Different phase II studies also investigated this agent in combination either with radiation or chemoradiation in advanced SCCHN. A randomized, phase II trial compared radiotherapy and nimotuzumab with radiotherapy and placebo in a group of 106 patients with unresectable SCCHN who were unfit for concurrent chemoradiation. Toxicities were limited to almost only grade I/II. The complete response rate was significantly higher in the nimotuzumab group (59.5%) versus the placebo group (34.2%; P = .038), as was survival ( P = .0491). However, the radiation dose used in this study was low and could be questioned. Another phase II study randomized 92 patients with (LA) SCCHN to receive radiotherapy alone or radiotherapy plus nimotuzumab (arm A) or chemoradiation without or with nimotuzumab (arm B). Locoregional response was better in the nimotuzumab arms: 76% versus 37% in arm A, and 100% versus 70% in arm B. The corresponding OS rates at 48 months were 34% versus 13% (nonsignificant) in group A, and 47% versus 21% in group B ( P = .01). The addition of nimotuzumab to chemoradiation resulted in a significant reduction in the risk of death by 65% (hazard ratio, 0.35; P = .01). These results should, however, be interpreted with caution owing to the low number of patients included.
Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
Reversible “selective” EGFR tyrosine kinase inhibitors, such as gefitinib and erlotinib, have been investigated in SCCHN ( Table 2 ).






