Epidermal Growth Factor Receptor (EGFR) Inhibitors
Timothy M. Dang
Daniela Kroshinsky
The human epidermal growth factor receptor (HER) family of proteins is normally involved in cell growth and survival.1 HER1, also known as epidermal growth factor receptor (EGFR), is ubiquitously expressed and regulates many cellular processes, including proliferation, apoptosis, adhesion, and motility.2 HER2 plays an important role in the pathogenesis of breast cancer, lung cancer, and other malignancies. The roles of HER3 and HER4 in cancer are not as well understood, though novel therapeutics against these specific receptors are an active area of investigation.3,4
Many malignancies contain mutations in the EGFR gene that render them susceptible to inhibition.5 Tyrosine kinase inhibitors (TKIs) against EGFR (hereafter referred to as EGFRi) are approved and used to treat non-small cell lung cancer, pancreatic cancer, colorectal cancer, head and neck cancer, and cutaneous malignancies. Drugs in this class include small molecule inhibitors, such as erlotinib, gefitinib, and osimertinib, as well as intravenous monoclonal antibodies, such as cetuximab, panitumumab, and necitumumab. Many TKIs, such as lapatinib and pertuzumab, block both EGFR and HER2. Trastuzumab is selective for HER2, though binding in the setting of HER receptor heterodimerization is possible.
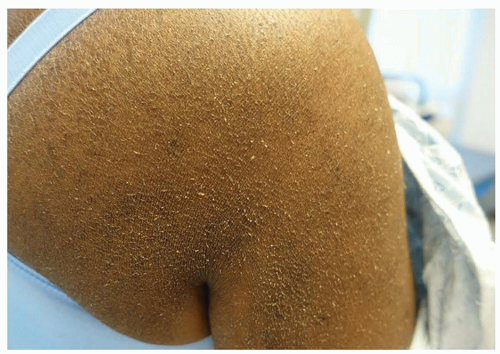
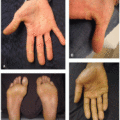
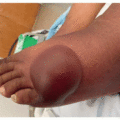
Dermatologic adverse effects (dAEs) associated with anti-HER therapy are due to on-target, off-tumor binding of EGFR in the skin, hair, and nails; osimertinib inhibits T790M-mutant EGFR and thus exhibits fewer and less severe dAEs than the EGFRis that inhibit wild-type EGFR, though the range of toxicities is similar. dAEs from these agents are predictable and often preventable and/or treatable. The most common specific toxicity associated with HER1/EGFRi is the characteristic papulopustular reaction with a predilection for the face and upper chest, affecting over 85% of patients on some EGFRis. Other expected dAEs include xerosis with disruption of the skin barrier such that dryness can be so severe that it leads to eczematous dermatitis (Figure 28.1), photosensitivity, hair changes, fissuring of the skin, and nail changes. Additionally, patients on EGFRis are prone to superinfection, primarily with Staphylococcus aureus.
There is a well-documented association between the presence and degree of rash, response rate to EGFRi therapy and overall survival; therefore, the severity of toxicity may be a surrogate marker for anticancer efficacy.6 Importantly, preventing or treating the eruption does not alter the associated survival benefit.
 Some studies have suggested that the incidence of rash from erlotinib in a cohort of Japanese patients was higher than overall incidence rates for all populations (93.4% vs. 70.4%)7.
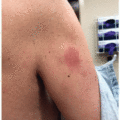
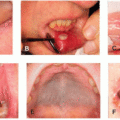
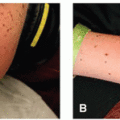
Some studies have suggested that the incidence of rash from erlotinib in a cohort of Japanese patients was higher than overall incidence rates for all populations (93.4% vs. 70.4%)7.The acneiform or papulopustular reaction from anti-HER therapy (particularly HER1/EGFRi therapy) has a predilection for the face, upper chest, and upper back.3,9 This rash develops in >50% of patients (and over 85% on some agents), usually within 2 to 4 weeks of initiating therapy. Although it can be self-limited, treatment is recommended because of the impact on patient quality of life; many patients report the sudden onset of this eruption as a privacy invasion as it is an outward presentation of the need for treatment for cancer. Manifestations include burning, irritation, and erythema in the early period, which then progress to a folliculocentric, papulopustular rash concentrated in seborrhea-rich areas (eg, face, upper chest, upper back) (Figure 28.2). Staphylococcal superinfection can lead to yellow crusting of the face, including the periorificial eyelid margins and nares (Figure 28.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree