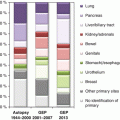
Metastatic sites
Start of follow-up
0 months+
3 months+
7 months+
Cases (%)
Cancer deaths
Median survival
Cases (%)
Cancer deaths
Median survival
Cases (%)
Cancer deaths
Median survival
Lymph nodes
576
434
8
405
286
15
292
185
28
Axilla
131 (3.7 %)
67
74
107 (6.2 %)
46
119
92 (10.0 %)
33
.
Head and neck
202 (5.7 %)
167
7
136 (7.8 %)
111
12
97 (10.5 %)
77
16
Extranodal
5576
4934
3
2824
2479
7
1523
1288
11
Liver
2650 (74.1 %)
2407
2
1121 (64.5 %)
1014
7
540 (58.4 %)
476
10
Bone
552 (15.4 %)
475
5
344 (19.8 %)
296
7
174 (18.8 %)
147
14
Mediastinum
40 (1.1 %)
33
7
31 (1.8 %)
25
14
21 (2.3 %)
16
19
These kinds of selections are also the likely reason for the apparent discourse between the autopsy-verified primary sites of CUP and those assigned by molecular methods (gene expression analysis) [14]. The latter tissue-of-origin assignments score much higher proportions of relatively benign cancers, particularly breast cancers, compared to autopsy data for which the lung and the pancreas dominate [15]. Many of the studies applying molecular methods used patient samples from referral hospitals.
2.3 Incidence
The CUP incidence variation may reflect the diagnostic trend, the completeness and validity of cancer registration, or the real variation in incidence in a group of heterogeneous cancers. CUP incidence rates ranging from 5 to 10 per 100,000 have been reported in Nordic countries during 1998–2002 when the incidence has been at its maximum [16, 17]. Van de Wouw et al. reported that approximately 2500 new patients were diagnosed annually in the Netherlands from 1984 to 1992, giving an age-standardized incidence rate of 6.7 per 100,000 for males and 5.3 per 100,000 for females [10]. The incidence of CUP is identical for men and women in Sweden, but in some countries, there is a small male or female excess [4, 12, 17]. Cases of CUP are diagnosed from age 30 onward with the peak age at diagnosis being in the 70s [17]. Brewster and coworkers discuss the sensitivity of the apparent incidence to the definition of CUP [4].
In the Nordic countries, CUP incidence increased until about 1995–2000, followed by a sharp decline which seems to be continuing [16, 17]. In the USA, the decline in incidence started already in around 1980 [12]. The decline in CUP incidence, which has been opposite to the incidence of all cancers, has implied that the proportion of CUP cases of all cancer has dropped from about 4–2 % [12, 16, 17]. Because of high fatality, CUP ranks the third or fourth among cancer deaths [4, 12]. In Sweden, the decrease in incidence has been noted for most metastatic locations but particularly for the liver [18]. The reasons for the decline are not known, but it is likely that new detection methods, including imaging techniques, have facilitated the detection of small dormant primary tumors. Diagnostic procedures have also become better defined and a CUP nowadays is really a CUP and not just an unspecified cancer. Another suggested possibility is that the decrease is the consequence of the declining incidence trends for some underlying primary sites of CUP, such as lung cancer [19]. However, the rate of decline in CUP incidence has been so fast that this latter contribution can only be a minor factor. CUP incidence trends were analyzed by birth cohort to detect differences among diagnostic periods [17]. The overall incidence trends were replicated in each birth cohort, suggesting minor birth cohort effects. The curves were similar for both sexes, providing further evidence, in view of the opposite lung cancer trends for men and women, for the overwhelming contribution of diagnostic methods to the recent decline in incidence.
2.3.1 Incidence in Immigrants to Sweden
Migrant studies comparing cancer incidence among immigrants to that in the native population are of great value in identifying environmental and genetic factors in cancer etiology [20]. The classical migrant studies on Japanese immigrants to the USA and on European immigrants to Australia have provided strong arguments for the predominant environmental etiology of cancer. Immigrant studies may also provide supplementary and confirmatory data on the incidence of cancer in indigenous populations, especially in countries with no reliable cancer registration [21]. Previous immigrant studies showed that cancer incidence among immigrants tended to approach that in the native population during the process of acculturation [21].
The incidence of CUP varies worldwide, even within ethnically homogeneous populations. The underlying reasons for these differences may include both genetic and environmental effects. Alternatively, the possible influence of differences in clinical and diagnostic performance, the frequency of autopsies, or the completeness and validity of cancer registration cannot be overlooked. The only identified immigrant study on CUP was conducted in Sweden, a country of a relatively high incidence of CUP [22]. Sweden has a covering cancer registration and a publicly funded health-care system. Immigrants account for 15 % of the population. All of this mark Sweden as an excellent place for immigrant studies. The authors hypothesized that exposure to risk factors early in life could impact on the occurrence of CUP, similar to experience with other cancers [21]. As all cancers are recorded in the nationwide cancer registry, differences in registration and coding practice can be minimized. The authors used a population-based cohort design to evaluate relative risks of CUP among first-generation immigrants, with a secondary aim being to estimate CUP incidence rates in the indigenous population.
The Swedish FCD was used to identify the study population, containing information on 1110 male and 1230 female CUP cases in immigrants compared with 13,949 male and 16,558 female CUP cases in native Swedes [22]. The median age at immigration was 28 for male CUP patients, 27 for female CUP patients, and 27 and 25, respectively, for all male and female immigrants. The median age at diagnosis of CUP was 64 for male immigrants and 67 for female immigrants, compared with 70 and 71, respectively, for male and female native Swedes. The average difference between age at immigration and age at diagnosis was over three decades for both sexes. Finns constituted the largest immigrant population. Standardized incidence ratios (SIRs) for CUP among immigrants varied considerably, probably reflecting the risk in the country of origin. The overall risk was significantly decreased (SIR = 0.88), especially in females (0.82 [22]). Reduced risks were observed in immigrants from a number of countries, including Finland (SIR = 0.89), Turkey (0.53), Iraq (0.51), Iran (0.33), Asian Arab countries (0.39), India (0.41), and East Asia (0.38) (both sexes analyzed together). Significant risk reductions were also observed among female immigrants from Germany (SIR = 0.76) and Greece (0.31). Conversely, risk of CUP was significantly increased among Danish immigrants. Increased risk was additionally observed among male immigrants from the Benelux countries (SIR = 1.67) and the former Yugoslavia (1.26). The increased or decreased CUP risks observed were concluded to suggest that early life environmental risk factors or genetic factors influence the development of CUP. A further resolution of the questions about causation awaits the second generation to be old enough to allow assessment of risks. The observed differences may give clues about incidence rates in countries of origin for which incidence data are lacking.
2.4 Risk Factors
Information on etiological factors and risk factors that contribute to the pathogenesis of CUP is extremely scarce. Smoking is the only established environmental risk factor for CUP [23, 24]. The risk of smoking in the Swedish CUP patients was 1.82-fold, substantially higher for CUP with respiratory system metastases (4.90) than for CUP with liver metastases (2.03) [23]. There was also evidence that body mass index was inversely related to CUP with liver metastases which the authors speculated might be due to alcohol abuse. In the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, a total of 651 incident CUP patients were detected [24]. Risk of being diagnosed with CUP was strongly related to smoking, with a relative risk of 3.66 for current, heavy smokers (26+ cigarettes/day) compared to never smokers. The data were adjusted for alcohol consumption, body mass index, waist circumference, and level of education. For alcohol consumption and level of education, weaker associations were observed but they were no longer statistically significant after adjusting for smoking and indices of obesity. The risk of CUP was increased by approximately 30 % for subjects in the highest versus lowest quartiles of waist circumference [24].
Low socioeconomic status and tobacco smoking-correlated occupations are risk factors for CUP; medical doctors had the lowest risk according to a Nordic occupational cancer study [25]. Poverty, race, and low educational level have been reported as risk factors in the USA [12]. In Scotland, the index of multiple deprivation almost doubled the risk [4]. This index is a weighted combination of data for income, employment, health, education, and access to services. The socioeconomic gradient is likely to be confounded by smoking at least to a certain degree.
We have unpublished data showing the association of both type 1 and type 2 diabetes with CUP. Familial risk is another established risk factor, discussed below.
2.5 Familial Risk
Family history has been fundamental to the understanding of heritable components in cancer and the discovery of cancer-predisposing genes. Even though high-risk cancer syndromes are rare, they have been important for the understanding of overall genetic mechanisms of cancer. We aimed, first, to estimate the occurrence of CUP in two or more family members, which would suggest a shared etiology for concordant disease. Then, familial clustering of CUP with any cancer was studied, with the hypothesis being that discordant associations may be informative for the site of the unknown primary tumor. A high discordant familial association between CUP and, say, lung cancer would suggest that lung cancer was the unknown primary site in the CUP patient.
Data from the Swedish FCD showed that 191 offspring diagnosed with CUP had family members who were also diagnosed with CUP [2]. Since a total of 6844 offspring were diagnosed with CUP, the familial cases of CUP accounted for 2.8 % of all cases. Altogether, offspring CUP patients had 5332 parents or siblings diagnosed with any cancer. Lung cancer was diagnosed in 512 offspring whose family members were diagnosed with CUP, these cases accounting for 3.1 % of lung cancer cases in the offspring generation, the highest proportion of all cancers. For any cancer, the proportion was 2.4 %. Age at diagnosis did not differ between familial and nonfamilial cases, with the exception of nervous system cancer and leukemia, the age differences for which were explained by the inclusion of more childhood cancers in the nonfamilial group. The total number of CUP patients was 35,168.
Familial risks were analyzed in three mutually exclusive proband groups defined by family history: parent only, sibling only, and parent and sibling [2]. The last group was referred to as multiplex families as multiple individuals were affected. As an example, a familial relative risk (SIR) of 2.00 for CUP would indicate that offspring of parents with CUP were twice as likely to be diagnosed with CUP compared to offspring whose parents were not diagnosed with CUP. Each of the proband types allowed an independent set of comparisons. When an increased SIR was seen in more than one comparison, a chance association was deemed to be unlikely. The SIR for CUP was 1.08 when only parents were diagnosed with CUP but was 1.69 when only siblings were diagnosed with CUP (p < 0.01). With regard to discordant associations, CUP incidence was increased in families with lung cancer cases: parent only SIR = 1.22, sibling only SIR = 1.76, and parent and sibling SIR = 3.29 (all p < 0.01). CUP incidence was also increased in families with liver cancer (SIR = 1.31 for parent only cases and 1.61 for sibling probands) and kidney cancer (SIR = 1.62 for siblings and 4.83 for multiplex families). The remaining CUP associations were found in only a single proband group: colorectal cancer in multiplex families (SIR = 1.86), when parents were diagnosed with upper aerodigestive tract (1.28) and bladder cancers (1.17), and when siblings presented with rectal (1.40) and breast (1.15) cancers. The essential results are illustrated in Fig. 2.1 by each proband type.
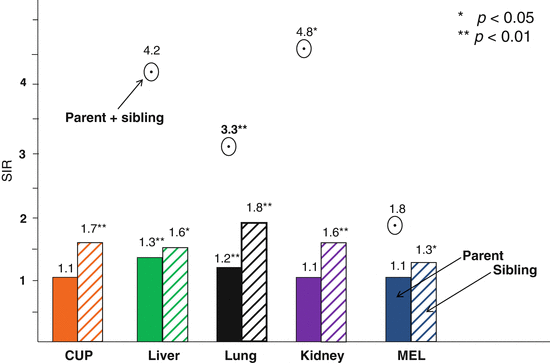

Fig. 2.1
Familial risk (SIR) for CUP when a parent (left bars), a sibling (right bars), or a parent and a sibling (round symbol) were diagnosed with the indicated cancers. The SIRs and their significances are shown on top of the bars or symbols. CUP patients had no affected parents + siblings (The source is Hemminki et al. [2])
Analyses were also carried out in reverse order, i.e., of cancer in offspring whose family members were diagnosed with CUP [2]. For parent–offspring relationships, the analyses were completely independent of the above data. For siblings, the SIRs for colorectal (1.26) and kidney cancers (1.82) increased. The SIRs for colon (1.12), pancreatic (1.26), ovarian (1.18), and prostate cancers (1.08) were significantly increased when the parents were diagnosed with CUP.
The results had several implications. In previous studies on discordant familial cancers, it has been consistently found that discordant familial risks are always lower, usually much lower, than concordant risks, implying that the genetic or environmental risk factors of familial clustering are strongest for a defined type of cancer [26]. The present results did not follow the established pattern. CUP showed no concordant familial clustering between parents and offspring, whereas many associations between CUP and discordant sites were significant between parents and offspring. A second inconsistency was that, even though the SIR for CUP among siblings was elevated to 1.69, it was lower than or equal to the associations between siblings for CUP–lung (1.76, 1.87), CUP–kidney (1.62, 1.82), and CUP–liver (1.61, 1.67) cancer pairs. A third deviation from the established pattern was that CUP was associated with high SIRs for multiplex families of colorectal (1.86), lung (3.29), and kidney cancers (4.83) in which a parent and at least one offspring were diagnosed with these cancers. Such high risks are best explained by CUP being highly related to these cancers, suggesting that the primary tumors originated from these organ sites. This reasoning should also explain the high risks among siblings in CUP–lung and CUP–kidney cancer pairs.
The familial associations suggest that the lungs, kidney, and colorectum are common but not exclusive sites of primary tumors, in line with the findings of autopsy studies [15, 27]. Interestingly, although molecular methods used in tissue-of-origin assignment studies have largely confirmed the autopsy results, they appeared to show increased frequencies for breast and bladder as sites of the primary tumors. In the present analysis, breast and bladder cancers were associated with CUP but each only in a single analysis.
Taken together, the family data have several implications. First, CUP is not a randomly occurring metastatic cancer; instead, it shows defined familial clustering. Second, familial clustering occurs between many cancer sites, the number of which may be limited by the statistical power of the present study. Third, the strong association of CUP with families of kidney, lung, and colorectal cancers suggests a marked genetic basis. Fourth, the involvement of many organ sites suggests that some mechanisms controlling defense against primary tumors and metastatic growth are shared by many cancer types. Fifth, the familial sites shared by CUP are likely to suggest sites of origin for CUP. Finally, a mechanistic exploration of CUP is a challenge, but it potentially offers rewarding insights into both host defense against primary tumors and the metastatic process in general.
2.6 Survival
The probability of survival after diagnosis of CUP was over 20 % at 12 months [17]. Survival by sex, tumor type, period of diagnosis, lymph node involvement, and age at diagnosis was explored separately. No sex difference was noted. Survival was worse in cases of adenocarcinoma and undifferentiated carcinoma compared with SCC and other histological types. Analysis of survival by period of diagnosis showed that those diagnosed in the 1960s had better survival compared to the other cohorts, which may reflect the low incidence of adenocarcinoma during the early part of the study period. Patients with nodal CUP persistently experienced a better prognosis than extranodal CUP cases: the probability of survival at 12 months in these two patient groups was around 70 % and less than 20 %, respectively. Age at diagnosis was significantly associated CUP patient mortality, with older patients showing worse survival. The recent data from Sweden and Scotland show minor improvements in survival, while the US data show none [4, 12, 28]. The Swedish data showed significant improvements for CUP with metastatic locations in the pelvis, peritoneum, and nervous system which were suggested to be due to therapeutic gains [28].
A detailed survival study of all Swedish CUP patients showed a large difference between histological types (Fig. 2.2) [11]. The survival curves for adenocarcinoma (8276 deaths) and undifferentiated carcinoma (2404 deaths) were almost superimposable, with a 12-month survival (shown by a vertical line) of 17 and 16 %, respectively. Half of the observed deaths occurred within the first 3 months after diagnosis, i.e., the median survival time was 3 months. The 12-month survival for squamous cell carcinoma (384 deaths) was 36 % and for melanoma (460 deaths), it was 51 %; the median survival times were 6 and 13 months. For CUP overall, the 12-month survival was 19 % and the median survival was 3 months.
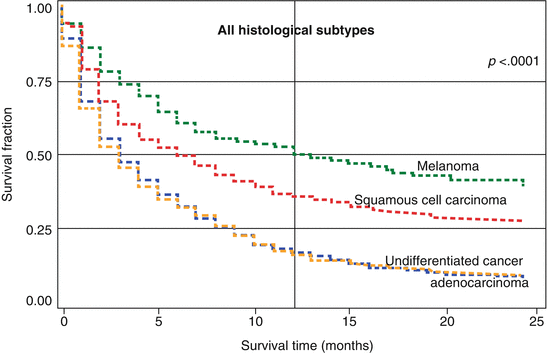

Fig. 2.2
Kaplan–Meier survival curves for CUP patients with extranodal metastasis by histology. The p-value in the top right corner refers heterogeneity of the survival kinetics (log-rank test) (Source Hemminki et al. [11])
In the same study, data for survival in adenocarcinoma and in undifferentiated carcinoma at extranodal sites were shown by location of metastases in the left and right panels of Fig. 2.3 [11]. Graphs for adenocarcinoma patients (left panel) show that the 12-month survival was lowest for patients with small intestinal (5 %) and liver (7 %) metastases. The 12-month survival for unspecified CUP (i.e., no location given) was 19 %; the survival curves for metastases in the peritoneum (12-month survival 17 %), other digestive organs and spleen (19 %), lung (20 %), and pleura (21 %) were almost superimposable with unspecified CUP and they were not shown. Bone adenocarcinoma showed also a 21 % survival fraction, skin 27 %, suprarenal gland and other sites 28 % (not shown), and brain 32 %. Patients with metastases in the ovaries (36 %), colorectum (40 %), and mediastinum (43 %) had the most favorable survival. For undifferentiated carcinoma (right panel), the survival was at least as poor as for adenocarcinoma for most metastatic locations; patients with mediastinal tumor masses survived best.
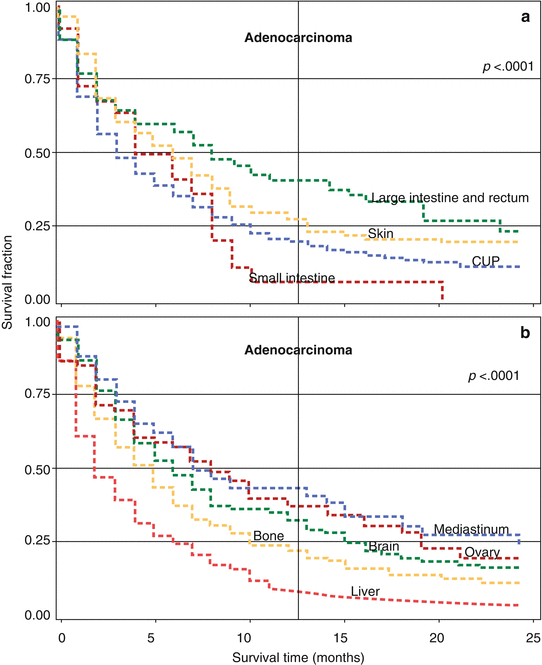
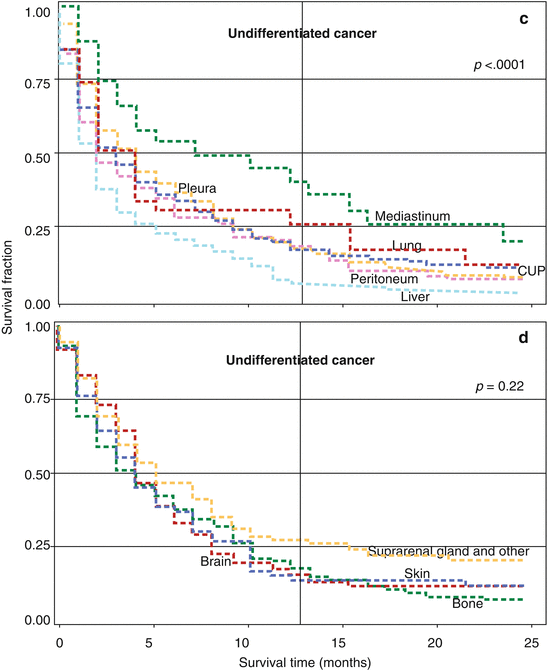


Fig. 2.3
Panels: (a, b) Kaplan–Meier survival curves for CUP patients with adenocarcinoma histology by metastatic location. Survival curves for cancer in the peritoneum, other digestive organ and spleen, lung, and pleura are not shown, but they are almost superimposable with unspecified CUP (shown by “CUP,” i.e., no specific location given). Panels: (c, d) Kaplan–Meier survival curves for CUP patient with undifferentiated carcinoma by metastatic location. The p-value in the top right corner of each panel refers heterogeneity of the survival kinetics (log-rank test) (Source Hemminki et al. [11])
When metastases were limited to lymph nodes, the 12-month survival was 41 % and median survival was 8 months [11]. Patients affected in the head and neck, axillary, and inguinal regions had the best prognosis and those with abdominal and intrapelvic metastases the worst prognosis. The published data underline the importance of histology and location of metastasis in assisting clinical decision-making: hazard ratios differed by a factor of five among extranodal and nodal metastases [11].
As no population-based survival data were available on CUP linking the location with the cause of death, we decided to investigate the issue [29]. However, basing analysis on the cause of death may be confusing because normally, the cause of cancer-related death in cancer patients is the primary cancer. However, CUP is an exception at least in Sweden because it is the only cancer for which death certificates give the site of fatal organ metastasis, as judged by the death registrar, as the cause of death. Death certificates are of high quality in Sweden because 85 % of cancer patients die in hospitals and, for over 90 % of cancer deaths, the related hospital records have been the basis on which the death certificate was issued [5, 29].
A total of 9300 CUP patients with extranodal metastases of adenocarcinoma and undifferentiated carcinoma were identified from the Swedish Cancer Registry. Lung cancer was the most common cause of death in patients with CUP metastasis in the respiratory system, nervous system, bone, and skin, with a median survival of 3 months. Patients with peritoneal/retroperitoneal and pelvical metastasis died of ovarian cancer, with a favorable median survival of 8 months. Patients with pancreatic, liver, biliary, and colorectal cancers with liver metastasis succumbed quickly. The data showed that the location of metastases predicts site-specific cancer deaths which may point to the hidden primary tumor. The diagnostic arsenal should be used to find lung tumors when metastases are diagnosed in the respiratory or nervous system, bone, or skin; ovarian tumors should be suspected after diagnosis of pelvical metastases [29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree