Fig. 28.1
US myeloma mortality rates (age-adjusted, 2000 U.S. standard) by race and sex, 1953–1957 to 2003–2006
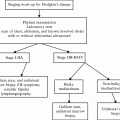
Rates specific for blacks, available since the early 1970s, are higher than rates for all nonwhites combined, and the rates increased more rapidly until peaking among males (9.5/100,000) and females (6.6/100,000) in 1993–1997.
To evaluate whether the increases in myeloma mortality during the past five decades were confined to certain age groups or specific time periods, rates were examined according to age group and year of birth (Fig. 28.2). For all race and gender categories, the increases in mortality occurred primarily among individuals aged 55 years and older, with the most marked changes occurring in the two oldest age groups, 75–84 and 85+. The risk of dying from myeloma rose steadily among individuals born in the mid to late 1800s and early 1900s. Whether the substantial rises among older persons born prior to the early 1900s were due to improving diagnosis or to increasing risk due to environmental exposures is not well understood. It is noteworthy that rates among middle-aged persons born more recently have not risen in a similar fashion. Over the past decade, rates declined in virtually all race/gender/age groups. Among those ages 35–54, the declines appear to have started among cohorts born since the 1940s.
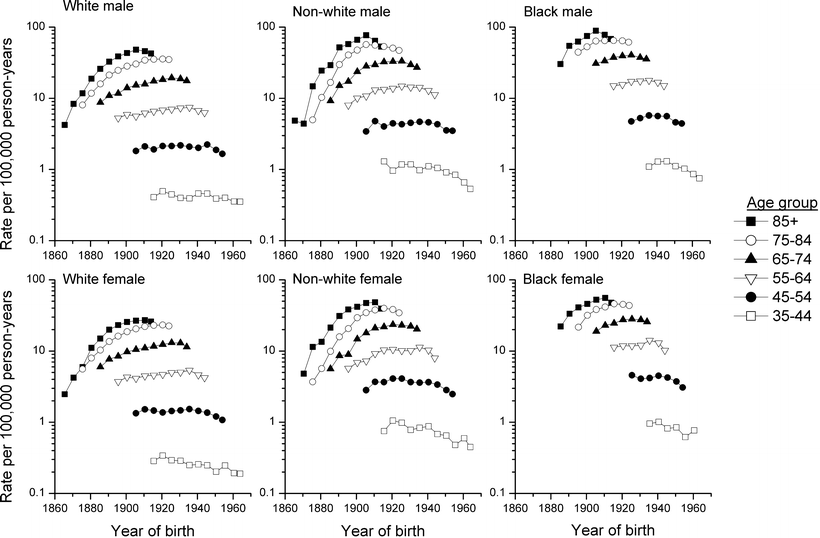

Fig. 28.2
US myeloma mortality by race, sex, age-group, and year of birth, 1860–1970
US Survival Patterns
Survival data based on follow-up of cases diagnosed since the 1970s are available from the nine population-based cancer registries of the Surveillance, Epidemiology, and End Results (SEER) program that included approximately 10 % of the US population [2]. Although survival among patients diagnosed with myeloma is poor for all race–gender groups, significant improvements in the 5-year relative survival rates have occurred over the past several decades, from 25.9 % among patients diagnosed during 1975–1977 to 37.1 % among those diagnosed during 1999–2005 (data not shown) [2].
The 5-year relative survival rates by age at diagnosis, race, and gender for patients diagnosed during 1999–2005 with follow-up through 2006 are presented in Table 28.1 [2]. For all race–gender groups, survival was more favorable among those diagnosed at younger ages than among the elderly. The 5-year relative survival rates for patients aged less than 45 were 55 % or greater, for those 55–64 ranged between 38 % and 46 %, and for those 75 years or older were 22 % or less. Survival rates for all ages combined were highest for white males (38.6 %) and lowest for white females (34.9 %), with rates among blacks intermediate the two.
Table 28.1
Five-year relative survival rates (percent) among patients diagnosed with myeloma in the SEER areas during 1999–2005 by age at diagnosis, race, and sexa
Whites | Blacks | |||
|---|---|---|---|---|
Age at diagnosis | Males | Females | Males | Females |
<45 | 63.6 | 57.3 | 55.2 | 58.9 |
45–54 | 54.9 | 55.2 | 41.2 | 52.2 |
55–64 | 46.2 | 44.9 | 38.7 | 37.7 |
65–74 | 32.8 | 34.8 | 31.0 | 32.7 |
75+ | 19.8 | 19.5 | 21.5 | 22.0 |
All ages | 38.6 | 34.9 | 36.0 | 37.2 |
Under 65 | 51.4 | 49.6 | 42.0 | 46.4 |
65 and older | 27.1 | 26.3 | 27.6 | 27.6 |
US Incidence Patterns
In contrast to the notable increases in mortality rates during 1953–1997, incidence rates (age-adjusted, US 2000 standard) rose more modestly in the nine SEER areas during 1973–1997 (Fig. 28.3). Rates were highest among black men, followed by black women, and then white men; they were lowest among white women. Rates among black men peaked at 14.8/100,000 in 1993–1997, and then declined to 13.9/100,000 in 2003–2006. Rates among black and white women also peaked in 1993–1997; the peak occurred among white men during 1998–2002. Data are available for Hispanics and Asian/Pacific Islanders in the SEER 12 since the early 1990s. Rates among males of each group also declined since 1993–1997, whereas among females they each peaked during 1998–2002. Rates have been consistently higher among Hispanics than Asian/Pacific Islanders.
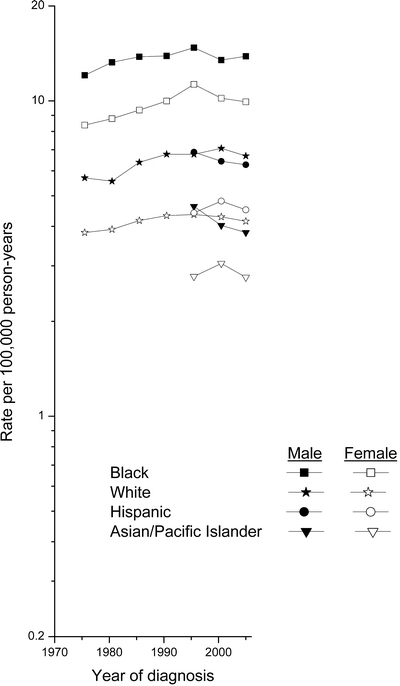

Fig. 28.3
Myeloma incidence rates (age-adjusted, 2000 U.S. standard) by sex among SEER 9 whites and blacks, 1973–1977 to 2003–2006, and among SEER 12 Hispanics and Asian/Pacific Islanders, 1993–1997 to 2003–2006
Age-specific myeloma incidence rates for 1993–2006 are presented in Fig. 28.4. Among all racial/ethnic/gender groups, rates rose exponentially with age over the adult age range, with the increases generally less rapid at the older ages. Rates were notably higher among blacks than whites and among males than females.
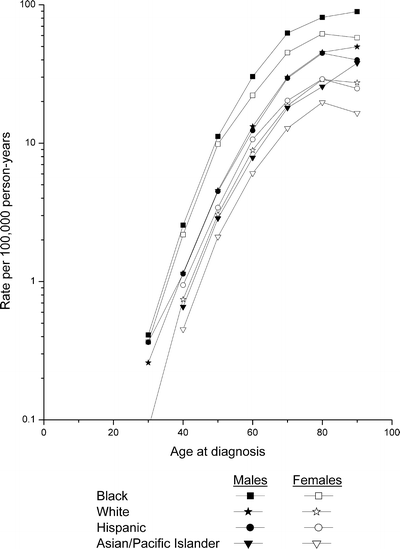

Fig. 28.4
SEER 12 age-specific myeloma incidence rates by race/ethnicity and sex, 1993–2006
For all racial/ethnic groups combined and individually, the incidence and mortality rates were 30–90 % higher in males than females with the sole exception of incidence among American Indians/Alaska Natives (Table 28.2) [2]. Incidence and mortality rates among blacks generally were about twice those among whites. Rates among Hispanics were very similar to those among whites, whereas rates among Asian/Pacific Islanders were substantially lower.
Table 28.2
Myeloma incidence and mortality ratesa by race/ethnicity and sex, 2002–2006
Race/ethnicity | SEER 17 incidenceb | US mortalityc | ||
|---|---|---|---|---|
Males | Females | Males | Females | |
All races | 7.1 | 4.6 | 4.5 | 3.0 |
White | 6.6 | 4.1 | 4.3 | 2.7 |
White Hispanic | 6.6 | 4.7 | 3.5 | 2.6 |
White Non-Hispanic | 6.6 | 4.0 | 4.3 | 2.7 |
Black | 14.3 | 10.0 | 8.2 | 5.8 |
Asian/Pacific Islander | 3.9 | 2.8 | 1.9 | 1.5 |
American Indian/Alaska Natived | 4.6 | 5.1 | 4.3 | 3.3 |
Hispanic | 6.4 | 4.7 | 3.4 | 2.6 |
International Patterns
International differences in multiple myeloma incidence, 1973–1977 to 1998–2002, as published in Volumes IV–IX of Cancer Incidence in Five Continents, are dramatic [3–8]. Generally, rates (age-adjusted, world standard) rose over the three decades among males in the US (SEER blacks and whites); Quebec and British Columbia in Canada; Finland; Denmark; the UK; Spain; and Osaka and Miyagi in Japan (Fig. 28.5) although in recent years the increases did not persist in several areas. For most countries, the time trends for females were similar to those for males. Among both males and females, the most rapid increases were in British Columbia, Spain, the UK, and Miyagi. Rates changed little in Norway, France, and Israel. In the Americas, rates have been consistently higher for SEER blacks than other populations, whereas rates have been the lowest in Colombia. Rates have been fairly similar and stable [9] in the Nordic countries, other European countries, and Oceania, although somewhat lower in Spain. In Asia, rates have been relatively low, except in Israel. Internationally, the lowest rates were in Shanghai, China. During the most recent time period, rates among US blacks were 7–8 times those in Shanghai.
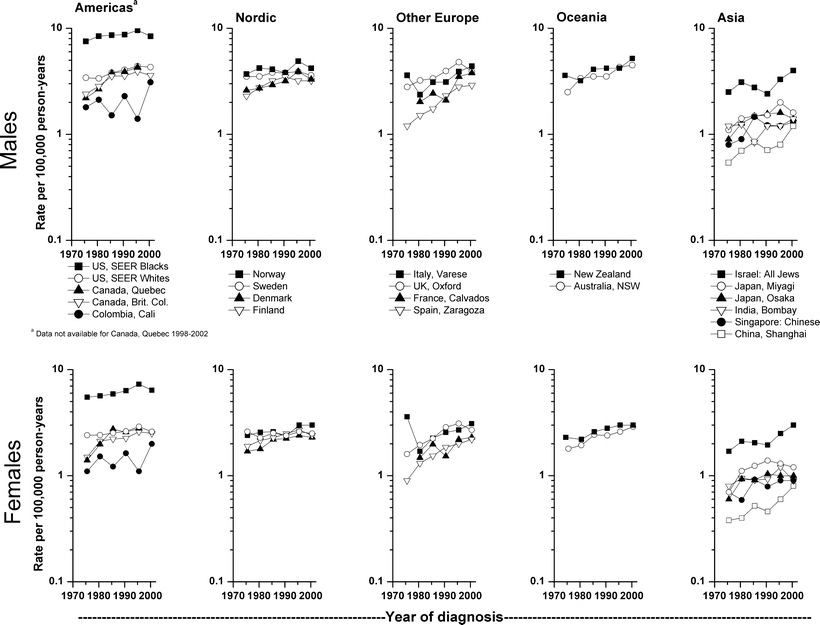

Fig. 28.5
International myeloma incidence rates (age-adjusted, world standard) by sex and continent, 1973–1977 to 1998–2002
Etiologic Factors
Ionizing Radiation
Most of the data on the effects of ionizing radiation on myeloma risk come from studies of Japanese atomic bomb survivors [10–12] and studies of therapeutically exposed populations [13–16]. However, myeloma also has been associated with lower levels of radiation exposure in several studies of occupational radiation exposure [17–21].
Atomic Bomb Survivors
Investigation of cancer incidence for the years 1950–1979 [10], and cancer mortality for the years 1950–1985 [11] among Japanese atomic bomb survivors suggested an increased myeloma risk with increasing radiation dose. However, a more recent analysis of the incidence data for the years 1950–1987 yielded an estimated absolute risk of 0.08 cases per 104 person years per Sv (95%CI <0–0.3), with no variation by gender, age at exposure, or time since exposure and no evidence of a significant dose–response relationship [12]. The most recent mortality analysis, covering the time period 1950–1990, yielded an estimated 0.17 excess risk per 104 person-years Sv (95%CI = 0.02–0.40) for both sexes combined. In a follow-up study of monoclonal gammopathy of undetermined significance (MGUS), subsequent multiple myeloma mortality was greater in the radiation-exposed subjects (2,284/100,000 person years) than in the total population (14.6/100,000 person years) [22]. Although the data available to date provide some support for an association between ionizing radiation from the atomic bombs and risk of myeloma, additional years of follow-up is required to reduce the statistical variability due to the small number of myeloma cases and elucidate the true nature of the association.
Diagnostic and Therapeutic X-Rays
Diagnostic X-ray exposure has not been clearly linked with myeloma. While several epidemiological studies reported no association between exposure to diagnostic X-rays and elevated risk of myeloma [23–28], other studies noted a positive association [29, 30].
Studies of the effects of therapeutic irradiation on myeloma risk have also been inconsistent. One case-control study has shown an excess risk of myeloma with radiation therapy [23] while others have not [16, 31, 32]. Elevated risks of myeloma were reported in follow-up studies of patients receiving X-ray treatment for ankylosing spondylitis [33, 34], cervical cancer [13], and metropathia hemorrhagica [14].
Radiation-Related Occupations
Studies of occupationally exposed individuals have provided additional information on ionizing radiation and risk of myeloma. An excess of myeloma deaths among American radiologists was first reported in the early 1960s [35]. In the 1970s, myeloma risk was found to be twice as high among US radiologists exposed to low-dose radiation than among physicians in other specialties [36]; however, more recently no excess of myeloma was reported in a large study of Chinese diagnostic X-ray workers compared to other medical specialists [37], or in a large population of US radiologic technologists [38].
Employment in nuclear facilities and risk of myeloma has been investigated in several cohort studies; elevated risks were observed in some, but not all. Small excesses of myeloma mortality were reported among employees of Sellafield in Britain [17, 39, 40] and Hanford in the USA [41], although the increase seen at Hanford did not reach statistical significance in a later follow-up [42]. An earlier combined analysis of cancer mortality data for over 95,000 nuclear industry workers employed at the USA (Hanford), UK (Sellafield), and Canadian nuclear plants found an almost two-fold significant increase for myeloma [19]. A 15-Country collaborative cohort study of over 407,000 nuclear industry workers also showed an association between low radiation and myeloma risk [21]. A borderline 60 % risk was observed among French nuclear workers [43]. Although a nested case-control study of workers employed at Hanford and Savannah River did not demonstrate an association between lifetime cumulative whole body ionizing radiation dose and myeloma, there was a significant effect of age at exposure, with positive associations between myeloma and doses received at older ages [20]. An apparent trend of increasing risk of myeloma mortality with increasing estimated external radiation dose, among workers included in the National Registry for Radiation Workers in the U.K., disappeared after excluding workers monitored for exposure to internal radiation emitters [44]. There was no evidence of increased risk of myeloma among nuclear workers in Belgium [45] or at the Chapelcross plant of British Nuclear Fuels [46].
Studies also evaluated the association between incidence and mortality of myeloma and uranium exposure. There was no apparent association among uranium miners in Czechoslovakia [47] or France [48] or among workers with chronic low-level exposure to internally deposited uranium at the Oak Ridge Plant in Tennessee [49]. However, two-fold significantly elevated SMRs were observed for myeloma among the Colorado Plateau uranium miners exposed to radon [50].
Although several follow-up studies evaluated the risk of myeloma mortality among nuclear test participants, there is no epidemiologic support for a strong association. Elevated risk of myeloma was observed in an early follow-up study of military personnel in the UK [51], but not in a later follow-up [52] nor among exposed military personnel in New Zealand [53] or the USA [54]. In addition, risk of myeloma among the UK test participants was nonsignificantly reduced [52].
Residential Exposures
A study in Spain reported an excess risk of myeloma mortality in the residential area near one of seven nuclear power plants [55]. However, little evidence of an increased risk of either myeloma incidence or mortality was noted in other investigations of the relation of residential proximity to nuclear facilities [56–60]. Similarly, there is little evidence for an increased risk of multiple myeloma mortality among populations living near uranium milling and mining operations [61, 62].
Occupational Exposures
Farming and Other Agricultural Occupations
The majority of epidemiologic studies that evaluated the risk of myeloma among farmers and other agricultural workers have reported positive associations [63–74]. A meta-analyses of 32 studies of myeloma and farming, published between 1981 and 1996, yielded an overall estimated relative risk of 1.23 (95 % CI = 1.14–1.32) [75]. Exposures commonly experienced by farmers that might contribute to the increased risk of myeloma include grain dusts [76, 77], engine exhausts and fuels [31], contact with farm animals [78–80], and pesticides [63, 69, 71, 81]. A number of epidemiologic studies have evaluated the use of specific pesticides some studies have noted positive findings with chlorinated phenoxy herbicides [78], insecticides as a group [81], DDT [78, 82], permethrin [83], glyphosate [84], and alachlor [85].
Metal Workers
Increased myeloma risk has been reported among workers in various metal occupations and industries [80, 86–92], although there are only limited data for exposure to specific metals [78, 93]. Significantly elevated risks have been observed among smelter and metallurgy workers [88], machinists [86], nickel refinery workers [89], sheet metal workers [80, 90], and metal processors [94]. In contrast, other studies have reported no notable associations with occupational metal exposures [95–97].
Rubber Manufacturing
Some epidemiologic studies have suggested an association between myeloma and employment in the rubber manufacturing industry [24, 98–103]. However, overall results from a recent review of 12 cohort studies in nine countries, seven industry-based case-control studies, 48 community-based case-control studies in 16 countries, and 23 studies based on administrative data, reported no excess risk for myeloma with employment in this industry [104]. In addition, myeloma was not found to occur in excess among workers exposed to styrene or butadiene in the rubber or the reinforced plastics and composites industry [105–109].
Wood Products Workers
Several studies reported associations between myeloma risk and employment in the wood, furniture, and pulp and paper manufacturing industries [91, 110–114]. Other studies showed little or no elevation in myeloma risk among wood product workers [88, 97, 115, 116], with the possible exception of forestry workers [78].
Other Industries and Occupations
An association between myeloma and employment in textile processing has been suggested in a few studies [90, 112, 117–119]. In contrast, two cohort studies of textile workers revealed no significant increase in deaths due to myeloma [120, 121]. Excess risks of myeloma among workers employed in the paint manufacturing industry have been noted in several studies [87, 122–125]. Other occupational groups linked with elevated risk of myeloma in at least one study include nurses [126], pharmacists and dietitians [80], science technicians [127], childcare workers [128], female workers exposed to silica [129], cosmetologists and hair dressers [130, 131], railway carriage construction and repair workers [132], roofers [80], meatcutters [133], tailors and firefighters [69, 134].
Specific Occupational Exposures
The relationship between benzene exposure and risk of myeloma remains controversial [135–137]. Although benzene was replaced decades ago by toluene or xylene in most developed countries, it still remains as a low-level contaminant in gasoline, other solvents, and in many products used or manufactured by the petroleum industry. Several studies have suggested benzene as a possible etiologic agent for myeloma [138, 139], and one study reported an excess of myeloma among women residing within 7.5 km of a petrochemical plant in South Wales [140]. In other studies, however, either the number of exposed myeloma subjects was too small to yield significantly elevated risk estimates, or there was no elevation in risk [141–146]. In addition, no excess risk of myeloma was reported in a recent meta-analysis of 22 cohort mortality studies of petroleum workers in the UK, Canada, the USA, and Australia [147].
Other Chemical Exposures
Excess myeloma mortality has been reported in some [148, 149], but not all [150–152] investigations of chemical workers. Elevated risk of myeloma was linked to high levels of dioxin exposure in a follow-up study among residents of Seveso, Italy [153], among fishermen residing on the east coast of Sweden [154], and among verified clusters of myeloma located near dioxin-contaminated bodies of water [155]. Exposure to organic solvents among aircraft maintenance workers and workers in the painting industry has also been linked to excess risk of myeloma [69, 122, 125, 156, 157]. While a meta-analysis of eight cohort or case-control studies showed no evidence of an association between trichloroethylene and myeloma risk [158], a recent large population-based case-control study suggested that exposures to certain chlorinated solvents may increase risk of myeloma [159]. A positive association between myeloma and asbestos exposure has been reported in some [25, 93, 160], but not all [24, 69, 116, 161] studies.
Lifestyle Factors
Cigarette Smoking and Alcohol Consumption
No relation with cigarette smoking or snuff use has been reported for most epidemiologic studies of myeloma [24, 74, 76, 93, 118, 162–170]. However, significantly elevated risks were noted in a cohort of Seventh Day Adventists [171], among women in the Third National Cancer Survey [172], for ex-smokers in a Swedish case-control study [31], and a small case-control study in former Yugoslavia [173]. No epidemiologic study has reported a significant positive association between myeloma and alcohol consumption [24, 74, 76, 93, 164, 174, 175]; a significant inverse dose–response relationship was observed in a recent case-control study in Connecticut [176].
Hair Dyes
There is conflicting evidence on whether personal use of hair dyes increases the risk of developing multiple myeloma [177]. Elevated risks of myeloma have been reported among women and men who used hair dyes in some earlier case-control studies [178–180], but not all [118, 179], with the greatest risk observed among those using permanent hair dyes and dark hair coloring products [180]. There was no overall increased risk of myeloma among women who used permanent hair dyes in the American Cancer Society prospective mortality study; however, an elevated risk was observed among women who used black hair dye for 20 years or more [181, 182]. Although the Nurse’s Health Study lacked information on color of hair dye used, no excess risk of myeloma among women whose natural hair color was dark brown or black was found [183]. Overall there was no evidence of an association between hair coloring product use and myeloma risk in several recent case-control studies [184–187]. In two of these studies [184, 185], however, significantly elevated risks were observed for certain kinds of hair dye use; semi-permanent dye in one study and black permanent dye by men (based on small numbers) in the other. There are also inconsistencies in the epidemiologic literature concerning the relation between myeloma and employment as a hairdresser, beautician, or cosmetologist, with excess risks reported in some studies [118, 130, 131], but not in others [112, 179, 188].
Obesity and Diet
Obesity has been identified as a risk factor for several cancers, and there is increasing evidence for its role in multiple myeloma. Several epidemiological studies have shown increased risk of myeloma in relation to obesity. A recent meta-analysis of body mass index (BMI) and multiple myeloma including 11 cohort and four case-control studies showed significantly elevated risk among overweight (BMI = 25–30 kg/m2) and obese (BMI > 30 kg/m2) individuals compared to those with normal BMI [189]. Per 5 kg/m2 increases in BMI, this meta-analysis reported a 1.14-fold (95 % confidence interval (CI) 1.09–1.20) risk based on nine cohort studies and a 1.43-fold (95 % CI 1.28–1.59) risk based on four case-control studies. Another meta-analysis of prospective observational studies published between 1996 and 2007 with some additional studies, found a 1.11-fold (95 % CI 1.05–1.18) risk for men and a 1.11 (95 % CI 1.07–1.15) risk for women per 5 kg/m2 increase in BMI [190]. Subsequent to these meta-analyses, a study conducted in the Swedish and Finish Twin Cohort Study found a 2.1-fold (95 % CI 1.1–3.7) risk of multiple myeloma among obese participants (≥30 kg/m2) compared to those within the normal BMI range (18.5–24.9 kg/m2) [191]. In addition, borderline significant associations were found in the Netherlands Cohort Study (per 4-kg/m2 increase BMI: RR = 1.13, 95 % CI 0.97–1.31) [192], and in the European Prospective Investigation into Cancer and Nutrition (EPIC) (RR = 1.52, 95 % CI 0.92–2.51) for male participants with a BMI ≥28.7 kg/m2 compared to <24 kg/m2; however, the association was null for women [193]. Furthermore, MGUS, a precursor to multiple myeloma, was 1.8 times higher in obese black and white US women compared to those with normal BMI [194]. The suggested association between obesity and myeloma, perhaps through a mechanism that promotes myelomagenesis (growth, survival and migration of malignant plasma cells) [194], is biologically plausible. Cytokine interleukin-6 (IL-6) has an essential role in the initial progression of myeloma cell tumors via the RAS-mitogen activated protein (MAP) kinase signaling pathway, and promotes myeloma cell survival through the regulation and expression of anti-apoptotic proteins [195]. Obese persons have higher levels of IL-6 [196], which may in part be linked to the higher levels of the adipokine leptin, which is produced in adipocytes and is higher in obese persons [197]. Insulin-like growth factor-I (IGF-I) is another important survival and proliferation factor for myeloma cells through the JAK/STAT pathway and MAP kinase phosphorylation [198–200]. IGF is closely linked to obesity and metabolic syndrome [201].
With respect to diet, protective effects have been reported in relation to frequent intake of vitamin supplements (especially vitamin C, vitamin D, and calcium), fish, whole grains, and green vegetables (notably cruciferous vegetables) [173, 176, 202–206]. Elevated risks have been reported for sources of animal fat, primarily liver and dairy products [173,176, 205].
Socioeconomic Status
The relation between socioeconomic status (SES) and myeloma, whether measured by occupation, income, or education, has been evaluated in a number of epidemiologic studies. Two recent population-based case-control studies and a nested case-control study reported elevated risks of myeloma associated with lower SES [24, 207, 208]. In contrast, earlier studies observed either no association or a positive association between myeloma and SES, possibly due to under ascertainment of the disease in lower SES populations [110, 172, 209–212]. While one US case-control study showed that the occupation-based low SES accounted about 50 % of the excess myeloma among blacks [207], a recent US study observed increasing risk with increasing SES among African-Americans [213], the SES measure, however, was created based on ecological census track data.
Medical Conditions and Medication Use
Monoclonal Gammopathy of Undetermined Significance
MGUS is an asymptomatic condition that typically precedes myeloma and is characterized by a serum monoclonal protein (M-protein) concentration of less than 30 g/L, and the presence of fewer than 10 % clonal plasma cells in the bone marrow without evidence of myeloma [214]. A recent nested case-control study of prediagnostic serum samples among participants in a US cancer screening trial showed that MGUS was present in nearly all patients prior to myeloma diagnosis [215]. The risk of progression of MGUS to myeloma is approximately 1 % per year [216]. In a large Swedish MGUS cohort followed for 15 years, the standardized incidence ratio for myeloma was 34.3 (95 % confidence interval = 24.8–46.2), compared to the general population [217]. The actuarial probability for malignant transformation in two large series of MGUS patients was 31 % at 20 years [218] and 40 % at 25 years [219]. There are currently no recommendations for use of any clinical agent in MGUS [214], but the MGUS patients are recommended to be monitored indefinitely [220]. Some of the established and suspected risk factors for myeloma are also found to be associated with MGUS. These factors include black race [194, 221, 222], family history of MGUS [223], history of autoimmune disorders [224], exposure to radiation [225], and pesticides [226].
Chronic Immune Stimulation
A number of epidemiologic studies have investigated the hypothesis that repeated or chronic stimulation of the immune system may lead to myeloma [227]. Risk of myeloma has been evaluated by assessing past history of individual immune-stimulating medical conditions, by examining categories of such medical conditions as autoimmune conditions and allergies, and by classifying medical conditions according to their biologically or immunologically related immune response mechanisms [228, 229]. While some studies have observed elevated risks for specific immune-stimulating medical conditions such as rheumatoid arthritis [23, 230], pernicious anemia [224, 231, 232], polymyositis/dermatomyositis [224], ankylosing spondylitis [224], and thyroid diseases [233], the results across studies have been inconsistent.
Infectious Agents
The role of viruses or other infectious agents in the etiology of myeloma is still unclear. It has been suggested that immune-compromised persons may lack the ability to fight off viruses or other infections [234]. Many AIDS-related cancers have been associated with specific human herpes virus infections [235] and human herpes virus 8 (HHV-8) has been detected in Kaposi’s sarcoma and primary effusion lymphoma [236]. An increased incidence of myeloma was recently reported among people with AIDS in two large registry-based studies [234, 237], and HHV-8 was found in the nonmalignant bone marrow dendritic cells of myeloma patients [238]. However, the role of HHV-8 in the pathophysiology of myeloma is controversial [236] because most investigators have failed to detect a high seroprevalence of HHV-8 antibodies in patients with myeloma [239–242]. Several reports linked myeloma to prior bacterial and viral infections [224, 230, 243]. These associations, however, are difficult to interpret. Although it is possible that bacterial and viral infections may be the triggering factors for the development of myeloma they may also be the result of diminished immune competence during the prodromal period of myeloma [224, 227].
Medication Use
Although significantly elevated risks of myeloma have been reported with use of laxatives, erythromycin, and mineral oil (in females, but not males) [93, 244, 245], there is little evidence to suggest that any particular over-the-counter or prescription drug plays an important role in the etiology of myeloma [23, 26, 244]. In a recent study, a reduced risk of myeloma was found among women who used antilipid statin therapy or estrogen replacement therapy, while an increased risk was observed among women who used prednisone, insulin or gout medication [230]. Interestingly, it has been suggested that statins could trigger apoptosis by blocking many signaling cascades in multiple myeloma and may be useful in molecular targeted therapy in the treatment of subsets of myeloma [246, 247].
Familial and Genetic Factors
Familial Aggregation
An inherited component to myeloma has been suggested by case reports of families with affected members, especially siblings, and by observations that persons with myeloma are more likely than others to have relatives with myeloma or hematolymphoproliferative (HLP) cancer [231, 248–255]. In case-control studies of myeloma, investigators found two- to six-fold excess risks of myeloma among subjects who reported having a first-degree relative with myeloma and twofold excess risks among subjects with a history of HLP cancer in a first-degree relative [248, 249, 251, 253]. In the one study that investigated racial differences for myeloma, risk estimates for having a family history of HLP cancer tended to be higher for African-Americans than Whites, although the difference in risks was not statistically significant [249].
Genetic Factors
Although the etiology of myeloma is unknown, there is growing evidence that certain cytokines including interleukin-6 (IL-6), IL-1β, IL-10 and IGF, play an important role in the growth of plasma cells and may be involved in the pathogenesis of myeloma [195, 256, 257]. A small number of studies have examined whether genetic polymorphisms in immunoregulatory genes and growth factors influence susceptibility to developing multiple myeloma, and associations with variants in IL-6, TNF, IL4R, FCGR2 and IGF have been reported in some [258–260], but not all [261, 262]. Associations with variations in genes regulating innate immunity were observed in a recent study for SERPINE1, CCR7, and HGF [263]. Genetic variation in cell cycle and apoptosis-related genes, specifically CASP9, has also been found to be associated with multiple myeloma susceptibility [264, 265]. Evaluation of risk of myeloma associated with variants in genes involved in metabolism and response to exogenous chemicals such as CYP1B1, CYP2C9, EPHX1, PON1, AHR, and NQO1 showed no association for any of these variants except CYP1B1 [266]. Despite a strong a priori hypothesis for some of the variants studied to date, the main limitation of the published reports was small sample size, which may have led to false positive and negative findings [267], and which require replication.
Recent studies have demonstrated that most, if not all, patients with myeloma exhibit chromosomal abnormalities, but no specific abnormality common to all myeloma patients has been identified [268]. Typical abnormalities include a rearrangement involving the IGH gene at 14q32, with reciprocal translocations most often involving 11q13 and 4p16; chromosomal loss, most frequently monosomy 13; and structural rearrangement of chromosome 1 [268–271]. Although p53 gene mutations have been reported among myeloma cell lines [272, 273], they are rarely found in myeloma patients and thus unlikely to play a major role as a tumor suppressor gene in myeloma development [273–275].
Conclusion
Myeloma is an intriguing malignancy. From an epidemiologic perspective, it is the only hematolymphoproliferative cancer, other than chronic myeloid leukemia and peripheral T-cell lymphoma [276, 277] that is characterized by higher incidence rates among blacks than whites. Little is known about its etiology or reasons for the black excess in incidence.
The strongest associations have involved exposure to ionizing radiation and organic solvents and chemicals, and employment in farming and agricultural occupations. Recent studies suggest that certain lifestyle and genetic factors, particularly low socioeconomic status, overweight and obesity, and familial aggregation of cancer may be linked to excess risk of myeloma. Exposure to cigarette smoking and alcohol use do not appear to be related to myeloma, and data on personal use of hair dyes remain inconclusive. Some attention also has been focused on viruses and other infectious agents, but their role in the etiology of myeloma remains unclear.
There is growing evidence that chromosomal abnormalities and genetic variation in genes producing certain cytokines may be involved in the pathogenesis of myeloma. Investigations of independent and joint effects of genetic and environmental and lifestyle factors need to be incorporated into future epidemiologic studies to better understand the complex relationships between multiple factors in the development of myeloma.
References
1.
2.
SEER Cancer Statistics Review, 1975–2006. Based on November 2008 SEER data submission, 2009. http://seer.cancer.gov/csr/1975_2006/.
3.
Waterhouse J, Muir CS, Shanmugaratnam K, Powell J. Cancer incidence in five continents, vol. IV. Lyon: IARC Scientific Publication No: 42; 1982.
4.
Muir C, Waterhouse J, Mack T, Powell J, Whelan S. Cancer incidence in five continents, vol. V. Lyon: IARC Scientific Publication No: 88; 1987.
5.
Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J. Cancer incidence in five continents, vol. VI. Lyon: IARC Scientific Publication No. 120; 1992.
6.
Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer incidence in five continents, vol. VII. Lyon: IARC Scientific Publication No. 143; 1997.
7.
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents, Volume VIII. Lyon: IARC Scientific Publications, No. 155;2002.
8.
Curado MP, Edwards B, Shin HR et al. Cancer incidence in five continents, Volume IX. Lyon: IARC Scientific Publications No. 160; 2007.
9.
10.
Ichimaru M, Ishimaru T, Mikami M, Matsunaga M. Multiple myeloma among atomic bomb survivors in Hiroshima and Nagasaki, 1950-76: relationship to radiation dose absorbed by marrow. J Natl Cancer Inst. 1982;69(2):323–8.PubMed
11.
12.
13.
Boice Jr JD, Day NE, Andersen A, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst. 1985;74(5):955–75.PubMed
14.
15.
16.
17.
Smith PG, Douglas AJ. Mortality of workers at the Sellafield plant of British Nuclear Fuels. Br Med J (Clin Res Ed). 1986;293(6551):845–54.CrossRef
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
Davis FG, Boice JD, Hrubec Z, Monson RR. Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res. 1989;49(21):6130–6.PubMed
28.
29.
30.
31.
32.
Wright JD, St Clair CM, Deutsch I, et al. Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer. 2010;116(10):2486–92.PubMed
33.
34.
35.
36.
Matanoski GM, Seltser R, Sartwell PE, Diamond EL, Elliott EA. The current mortality rates of radiologists and other physician specialists: specific causes of death. Am J Epidemiol. 1975;101(3):199–210.PubMed
37.
38.
39.
40.
Omar RZ, Barber JA, Smith PG. Cancer mortality and morbidity among plutonium workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer. 1999;79(7–8):1288–301.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree