BACKGROUND
Historically, the study of diabetes in pregnancy has focused on either women with Type 1 diabetes, whose poor obstetric outcomes once led to an editorial entitled “They give birth astride the grave”1 or GDM, an entity which remains contentious,2,3 with variable approaches to definition, screening, and diagnosis.4 The current epidemic of obesity and diabetes among children, adolescents, and non-pregnant adults5 has changed the situation, leading to growing numbers with Type 2 diabetes in pregnancy and GDM (including undiagnosed Type 2 diabetes).6–8 In parallel, our understanding of the impact of diabetes in pregnancy for future generations,9 and our increasing ability to reduce pregnancy complications,10 and postpone, if not prevent, diabetes after GDM,11 have emphasized the epidemiologic and public health importance of diabetes in pregnancy.
OUTCOMES FROM DIABETES IN PREGNANCY
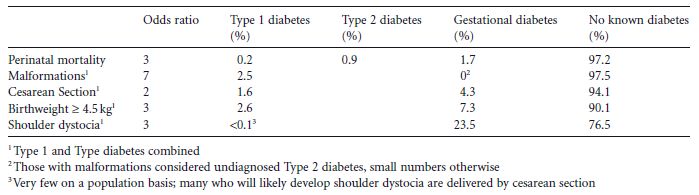
That there is no common unique pathognomonic complication of diabetes in pregnancy, combined with the apparent continuous relationship between glucose and fetal macrosomia, has resulted in a lack of consensus on the diagnosis of GDM. While diabetes in pregnancy is associated with increased obstetric risk compared with normal pregnancy, the overall contribution of diabetes to most obstetric and neonatal complications on a population basis is actually relatively low, with the largest impact being on shoulder dystocia (through GDM). Table 1.1 shows examples of odds ratios for each obstetric and neonatal complication by diabetes type and the proportion that diabetes in pregnancy contributes on a population basis.12–15
Apart from malformations, which are likely to have resulted from preconceptional or periconceptional hyperglycemia, improvements in obstetric practice have led to major reductions in adverse outcomes. Avoidance of such outcomes may dictate the need for complex obstetric decision-making, with the inevitable increase in fetal monitoring (see chapter 12) and which is strongly influenced by the preconceptional and antenatal management of hyperglycemia (see chapters 8 and 10). The importance of other metabolic factors, such as obesity16–19 and hypertriglyceridemia,20 in pregnancy are also now increasingly being recognized.
Table 1.1 Examples of odds ratios of diabetes for each obstetric and neonatal complication and the proportion that diabetes in pregnancy contributes on a population basis.12–15
The long-term implications of diabetes in pregnancy for the offspring, particularly obesity and Type 2 diabetes, are discussed in chapter 25. While there is early evidence that optimal management of diabetes during pregnancy may reduce excess adiposity in the offspring (and hopefully, ergo, subsequent diabetes),21 this urgently requires confirmation. As yet there is no evidence that poorer neurodevelopmental outcomes, which may be associated with GDM, are amenable to change.22 Such analyses can be confounded by the associations between socioeconomic status and both GDM and achievement.23
DIAGNOSIS OF GESTATIONAL DIABETES MELLITUS
While it is generally accepted that severe hyperglycemia in pregnancy is associated with adverse maternal fetal outcome, the significance of lesser degrees of hyperglycemia, along with the lack of common pathognomonic sequelae, and the apparent continuum between glucose and, for example, fetal macrosomia24 have fuelled the lack of consensus on the optimal glycemic threshold for diagnosis of hyperglycemia in pregnancy. This is discussed in chapter 6, but essentially involves deriving a glycemic threshold above which the benefits of intervention outweigh any harm and are cost-effective.
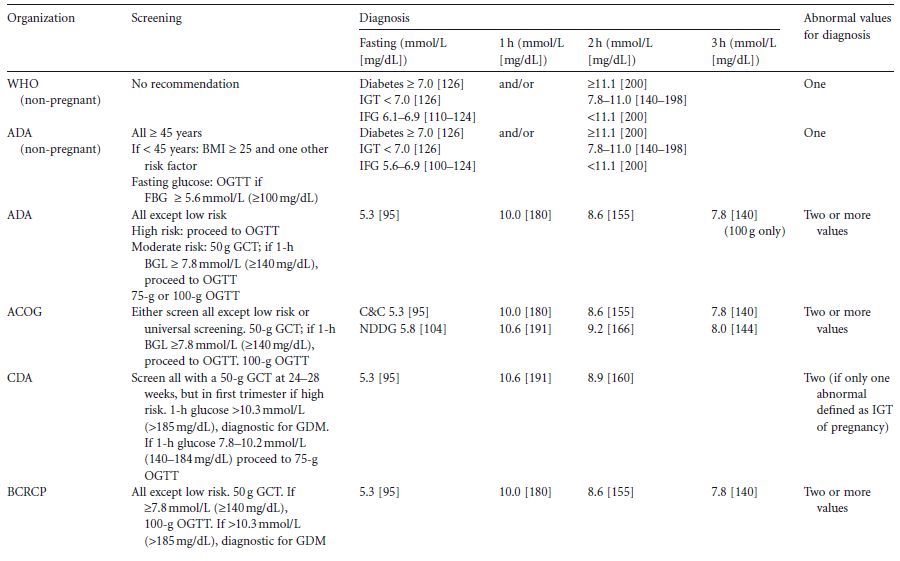
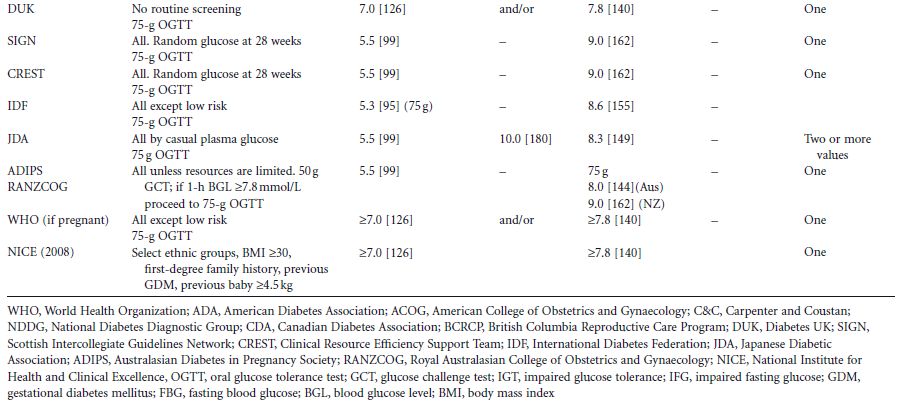
Outside of pregnancy, the 75-g 2-hour oral glucose tolerance test (OGTT) is used. Diabetes and prediabetes are defined by their association with macrovascular and microvascular complications, the clinical appearance of the latter (retinopathy) being largely unique to diabetes. As a result, diabetes in non-pregnant adults has a globally agreed definition, as does impaired glucose tolerance (IGT) (Table 1.2). There remains disagreement between the World Health Organization (WHO) and the American Diabetes Association (ADA) definition of impaired fasting glucose (Table 1.2). As GDM is defined as carbohydrate/glucose intolerance first identified/with new onset in pregnancy, intuitively it would be thought that by definition, the criteria for diagnosis of GDM would include a fasting glucose of greater than or equal 5.6 or 6.1 mmol/L (≥101–110 mg/d L) (ADA or WHO) and/or a 2-hour glucose greater than or equal to 7.8mmol/L (140mg/dL), with potentially some modification should pregnancy outcomes be quantitatively worse below these cut-off points. This is discussed more fully in chapter 6.
There have been multiple attempts to define the glycemic thresholds for fetal and maternal outcomes (i.e. diagnostic criteria) for GDM (Table 1.2). These have traditionally been based upon a fasting blood glucose test, 50–100-g glucose load,4 followed by 1–3 hours of blood glucose testing, and involving interpretation of the results either singly or in combination.
A global move to standardize the diagnostic criteria was the rationale for the Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO) study, a large study among 25000 women across continents and, importantly, involving many ethnic groups.24 Of importance is the fact that this study showed that the impact of hyperglycemia for maternal/fetal outcome was applicable to all ethnic groups24 and independent of maternal obesity, a recognized risk factor per se for large babies.16–19 Further analysis of data from the HAPO study will address the important question of whether different glycemic thresholds are needed to predict a greater risk of glucose-sensitive adverse outcomes.
Table 1.2 Summary of international guidelines for the screening and diagnosis of gestational diabetes (primarily from Cutchie et al4 )
PREVALENCE OF PREGESTATIONAL DIABETES IN PREGNANCY
The prevalence of Type 1 and Type 2 diabetes in pregnancy would be expected to reflect the rates of diabetes in the background population.25,26 However, the standard fertility ratio (SFR) is low in Type 1 diabetes (0.80, 95% CI 0.77–0.82), and is particularly low among women with retinopathy, nephropathy, neuropathy, or cardiovascular complications (0.63, 0.54, 0.50, and 0.34, respectively).27 While fertility rates in Type 2 diabetes have not beenreported, they would also be expected to be low (particularly in view of the additional associated obesity, polycystic ovarian syndrome [PCOS], and vascular disease).
The incidence of Type 1 and the prevalence of Type 2 diabetes has been increasing over time,28 with a reduction in the age at diagnosis of Type 2 diabetes. Both of these factors predict an increasing number of women with pregestational diabetes. However, the more rapid increase in Type 2 diabetes in pregnancy has resulted in some diabetes clinics now seeing a predominance of Type 2 over Type 1 diabetes, which has been accentuated further by ethnicity. In the US, the ratio of women with Type 1 to Type 2 diabetes has shifted from 3:1 to 1:2 between 1980 and 1995.28 This may be partly due to changes in the population (e.g. in Birmingham, UK the ratio of Type 1 to Type 2 diabetes was 1:2 in South Asians but 11:1 in Europeans29). Meanwhile, there have been other important changes. Women with diabetes in pregnancy are now expected to survive. The perinatal mortality for pregnancies complicated by Type 1 diabetes has also dropped from 40% to much nearer the background rate.23,28 In Type 2 diabetes, the evolving evidence suggests that perinatal mortality and the frequency of congenital malformations are similar to those of Type 1 diabetes,23 including in those women diagnosed with GDM but found to have Type 2 diabetes postnatally.6,29 While these trends are more often seen in women of non-European descent, it is likely that a similar picture will be seen in all groups eventually.
To date there are few reports of the prevalence of monogenetic forms of diabetes or secondary diabetes in pregnancy. Glucokinase mutations are present in up to 5–6% of women with GDM and up to 80% of women with persisting fasting hyperglycemia outside pregnancy, a small glucose increment during the OGTT, and a family history of diabetes.30 Cystic fibrosis is associated with a doubling in the prevalence of diabetes outside of pregnancy, with a further increase during pregnancy (e.g. from 9.3% at baseline to 20.6% during pregnancy, and 14.4% at follow-up).31
PREVALENCE OF GESTATIONAL DIABETES
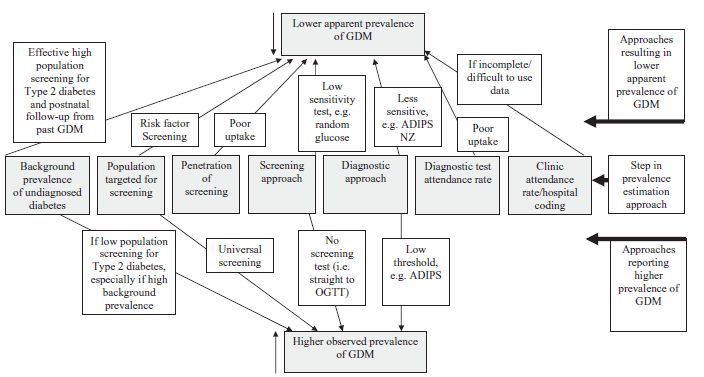
The prevalence of GDM globally in 1998 was examined by King et al.32 An epidemiologic comparison between studies is difficult for the reasons shown in Fig. 1.1 and discussed more fully in chapters 5 and 6. In addition, screening too early (before 24 weeks) will result in fewer cases of GDM being detected. Some women achieve the criteria for GDM only later in pregnancy and will not be diagnosed with the conventional screening approach, which occurs between 24 and 28 weeks.
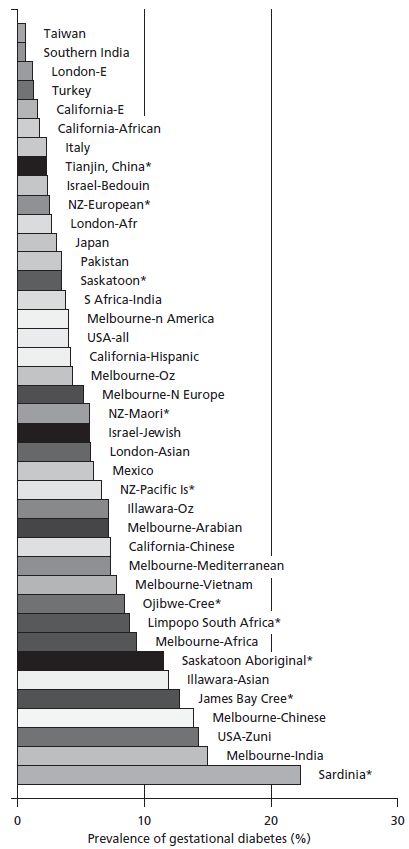
As highlighted above, there are differences in the rates of GDM when diagnosed by different criteria,33 both within and between populations (Fig. 1.2). Notwithstanding the different diagnostic criteria, there are major differences in prevalence of GDM between ethnic groups, reflecting both the background prevalence of Type 2 diabetes and the age at onset (the “underwater volcano hypothesis” [Fig. 1.3]).34 This hypothesis proposes that GDM is more common in people who are temporally closer to developing Type 2 diabetes.
These prevalence rates vary within the same ethnic group in different locations, with migrant populations generally having a higher prevalence than those remaining in traditional rural areas, probably relating to lifestyle change (higher energy diet, less physical activity) and greater adiposity. The prevalence has also generally increased over time (Fig. 1.4).7,35 While this most likely reflects the epidemics of obesity and Type 2 diabetes in the non-pregnant state, an additional feature is likely to be the increasing age at which pregnancy occurs, and for some total populations, the immigration of high-risk ethnic groups (e.g. in Auckland, New Zealand, numbers of women with GDM doubled over 4 years due to a combination of these factors36). Such data need careful scrutiny to recognize these factors and to ensure that no change in ascertainment (e.g. screening approaches) or diagnostic criteria have occurred.
All populations apart from those of non-European descent (and even including some European populations), are now considered at such high risk that most guidelines suggest that these ethnic groups require universal screening.4 The growth and clinical importance of undiagnosed Type 2 diabetes in pregnancy (i.e. the high end of GDM) also supports a universal screening approach in these populations, both at first antenatal assessment and at the more traditional 24–28 weeks of gestation. With the growing numbers of women with GDM, including undiagnosed Type 2 diabetes in pregnancy, the case for universal screening is becoming persuasive.37 Prior to the most recent data, the 4th Chicago Workshop on GDM38 recommended screening of all but those at very low risk (i.e. under 25 years, slim, no risk factors), a group of women who are becoming increasingly uncommon in modern pregnancy clinics. Surprisingly, during an obesity and diabetes epidemic, the latest recommendations from the National Institute for Health and Clinical Excellence (NICE) in the UK,39 recommend screening using very few risk factors, even excluding older women and those with PCOS from those warranting screening. Decisions underlying these recommendations have been informed by erroneous economic analyses, including the exclusion of (1) identifying women at high risk of future diabetes who could benefit from diabetes preventative intervention, (2) the benefits in the offspring from reduced exposure to maternal hyperglycemia, and (3) future pregnancies complicated by undiagnosed diabetes, as well as an underestimate of reduced benefits when using complex screening approaches.
Fig. 1.1 Difficulties in comparing prevalence data in gestational diabetes mellitus (GDM) with different approaches (personal observations). ADIPS, Australian Diabetes in Pregnancy Society.
Risk factors for gestational diabetes mellitus
While obesity, ethnicity, maternal age, and a family history of diabetes are the major risk factors for GDM, other more traditional factors have been used in selective screening approaches40 (Table 1.3), including parity and a previous macrosomic baby. Some studies have suggested that multifetal pregnancies (e.g. twins and triplets) may be at increased risk of GDM, although others have not confirmed this.40 There is increasing evidence of the importance of PCOS as a risk factor for both GDM and undiagnosed Type 2 diabetes in pregnancy. It therefore has been suggested in some countries that prior to treating PCOS with, for example, metformin or clomiphene to assist conception, women should have an OGTT.41
Another group of women at risk of GDM are those with a previous history of GDM,42 particularly in association with excess weight/weight gain between pregnancies and where previous GDM was diagnosed early in pregnancy and required treatment with insulin.43
Fig. 1.2 Prevalence of gestational diabetes in different populations at different times (* since King 1998,32 includes63–70).