9 Paul T. Strickland and Thomas W. Kensler History of Identification of Human Carcinogens • The carcinogenic effects of many environmental and occupational agents were first described in humans. • Beginning in the 20th century with the advent of animal bioassay programs, evidence of carcinogenicity in experimental animals has preceded evidence from epidemiological or case studies in humans. • Most human cancers probably result from the interaction of several or more carcinogenic influences (often unidentified) along with intrinsic factors (e.g., inherited genes, hormones, and immune status). Role of Environmental Agents in the Etiology of Human Cancer • Although the causes of many human cancers remain unidentified, cumulative data support the opinion that environmental or dietary agents are the principal cause of human cancers. • Cigarette smoking could be responsible for 25% of all cancers in the United States. • Chemical carcinogens include aromatic amines, benzene, aflatoxins, tobacco chemicals, and chemotherapeutic agents. • Radiation carcinogens include ultraviolet radiation, ionizing radiation, and radon. • A number of metal carcinogens have been identified, including arsenic, nickel, cadmium, and chromates. These carcinogens have been associated largely with occupational exposures. • Fibers (e.g., asbestos and silica) and dusts are well established as etiologic agents in lung cancers and mesothelioma. • Many components in the diet can influence the development of cancer through carcinogenic or anticarcinogenic mechanisms. Exposure Biomarkers, Susceptibility Factors, and Chemoprevention • The identification of molecular biological markers of exposure, effect, and susceptibility (reflecting events before clinical disease) will help further our understanding of human carcinogenesis. • The characterization of the human genome has permitted study of the roles of common polymorphisms in carcinogen metabolism or of DNA repair genes in susceptibility to cancer. • Primary and secondary approaches to the prevention of cancer will be greatly facilitated by the development of noninvasive biomarkers that identify high-risk individuals. • Tertiary prevention also might be enhanced by characterizing cancers with respect to etiology, genetic profile, or metabolic capacity. The carcinogenic effects of a sizable number of environmental or industrial chemicals have first been described in humans. The influences of occupation and lifestyle in cancer occurrence were observed at least as early as the 16th century. Ramazzini in 1700 noted that nuns showed a higher frequency of breast cancer than was observed among other women. Also in that century, Paracelsus and Agricola described “Bergkrankheiten” in miners in the Schneeberg and Joachimstal regions of Europe. Bergkrankheiten was later recognized as lung cancer, probably caused by uranium and its decay product radon.1 Subsequently, in 1761 Hill associated the use of tobacco snuff with cancer in the nasal passage, and in 1775 Pott noted the occurrence of soot-related scrotal cancer among chimney sweeps. In 1895 Rehn published evidence that occupational exposure to aromatic amines was associated with bladder cancer, and Unna in 1894 associated sunlight exposure with skin cancer. Contrary to experiences in earlier centuries, with the advent of animal bioassay programs, evidence of carcinogenicity in experimental animals has preceded evidence obtained from epidemiological studies or case reports in many instances. Although the term carcinogen means “giving rise to carcinomas” (e.g., epithelial malignancies) in general, broader operational definitions are used for carcinogens in animal bioassays. A carcinogen may be defined as an agent whose administration to previously untreated animals leads to a statistically significant increased incidence of malignant neoplasms, compared with the incidence in appropriate untreated control animals, whether the control animals have a low or high spontaneous incidence of the neoplasms in question. Chemicals, radiation, and infectious agents are the primary agents identified. Synthetic and naturally occurring chemicals compose the largest group of known human carcinogens. More than 100 chemicals, chemical mixtures, biological agents, physical agents, or industrial processes have been classified as Class 1 human carcinogens (Table 9-1) by the International Agency for Research on Cancer (IARC), and more than 300 chemicals have been designated as probable (Class 2A) or possible (Class 2B) human carcinogens. These figures evolve from an environmental milieu of perhaps 10 million chemicals, although the vast majority of these agents have not been evaluated for carcinogenicity. Table 9-1 Agents and Processes Considered Carcinogenic in Humans (Class 1) by the International Agency for Cancer Research Data from the International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risk to humans, vols. 1–104. Lyon (France): IARC; 1970-2012. An updated listing of the overall evaluation of carcinogenicity to humans can be accessed at http://monographs.iarc.fr under the heading “classifications.” (Note: For examples of carcinogenic ionizing radiations, see Table 9-3.) • Although the overall incidence of all cancers is reasonably constant among countries, incidences of specific cancer types can vary up to several hundred fold. • Large differences in tumor incidence exist within populations of a single country. • Migrant populations assume the cancer incidence of their new environment within one to two generations. Although the extent to which environmental agents contribute to human carcinogenesis remains to be defined precisely, a considerable number of epidemiologic studies indicate important roles for the various naturally occurring and manufactured chemicals, radiations, metals, and fibers found in our individual environments. Biological factors such as viruses (e.g., hepatitis B virus and human papilloma virus) and bacteria, elements of our “environment,” are also exceedingly important contributors to the human cancer burden. Biological factors are discussed in Chapter 11. The English surgeon Percival Pott was among the first to document the association of an environmental agent with cancer.2 During the late 18th century, he determined that the unusually high incidence of scrotal cancer among chimney sweeps was due to their occupational exposure to soot and tar. As a consequence, recommendations for bathing and use of protective clothing were promulgated by chimney sweepers’ guilds in parts of Europe, but not in England. Subsequent decreases in the incidence of scrotal cancer were observed in continental Europe, demonstrating the efficacy of simple prevention efforts. It was not until the present century that the active carcinogens in soot and coal tar were shown to be polycyclic aromatic hydrocarbons (PAHs).3 This finding came about through the application of coal tar and fractions thereof to the skins of test animals, in which malignant skin tumors subsequently developed. Although many PAHs were identified in coal tar, most of the carcinogenic activity was attributed to the PAH benzo[a]pyrene. Humans are exposed to PAHs from a variety of sources that include occupation, smoking, diet, and air.4 PAHs are readily absorbed into the body through the skin, lungs, and gastrointestinal tract. Occupational and medicinal exposures constitute the highest levels of human PAH exposure (albeit in small groups within the population), whereas diet and smoking are the major sources of exposure to PAHs in the general population. Air concentrations of greater than 10 µg benzo[a]pyrene/m3 are characteristic of topside gas and coke work environments. Broiled, barbecued, or smoked meats and fish contain relatively high concentrations of benzo[a]pyrene (1 to 20 µg/kg). Cutaneous occupational exposure to PAHs has been associated with increased risk of skin and scrotal cancers in chimney sweeps and in persons exposed to unrefined lubricating oils in the textile and machining industries.1 Scrotal cancer among mule spinners in the Manchester (United Kingdom) cotton industry was attributed to the saturation of the workers’ trousers with lubricating oil. A review of all admissions for scrotal cancer to the Royal Manchester Infirmary from 1902 to 1922 indicated that 49% had worked as mule spinners, whereas 16% had worked with tar or paraffin. As the textile industry declined in the middle 20th century, an increasing proportion of scrotal cancer was associated with cutting oils used in metal machining. An excess of lung cancer has been demonstrated among persons with substantial inhalation exposure to PAHs, including roofers and pavers, coke oven workers, certain steel and iron manufacturing workers, and aluminum production workers.5 In addition, several studies suggest that workers highly exposed through inhalation also might be at increased risk of cancer at sites other than skin and lung. The strongest evidence for such an association is for bladder cancer, where a dose-response relationship has been demonstrated between PAH exposure and bladder cancer risk in aluminum workers after adjustment for smoking. Other sites with suggestive increases in risk include the pancreas and upper gastrointestinal tract. Several biochemical pathways are involved in the metabolism of PAHs and of benzo[a]pyrene in particular.3 One major pathway results in highly reactive 7,8-dihydrodiol-9,10-epoxide-benzo[a]pyrene. Experimental studies demonstrate that cultured human lung or colon tissue metabolizes benzo[a]pyrene to reactive metabolites that bind to DNA in cultured tissue. Oral administration of benzo[a]pyrene to rodents produces benzo[a]pyrene-DNA adducts in liver, stomach, colon, and intestine, and cancers of the esophagus, forestomach, intestine, lungs, and mammary gland. The occurrence of bladder cancer among dye industry workers was reported in 1895 by the German physician Ludwig Rehn, who suggested a causal relationship. With the rapid expansion of the chemical industry during and after World War I, increased risk of bladder cancer was observed among workers employed in chemical manufacturing and textile dyeing.6 An industry-wide study of workers exposed to dyes in England and Wales demonstrated increased risks of bladder cancer among men exposed to 1-naphthylamine (observed [O]/expected [E] = 8.6), benzidine (O/E = 13.9), 2-naphthylamine (O/E = 86.7), or mixed dyes (O/E = 54.7). IARC subsequently considered that the cancer hazard associated with exposure to 1-naphthylamine was due to the probable contamination of commercial-grade 1-naphthylamine with 4% to 10% 2-naphthylamine.7 Additional studies in the dye industry identified auramine O and magenta as human bladder carcinogens. Increased risk of bladder cancer among rubber workers and in the electric cable industry has been attributed to the naphthylamine added to rubber as an antioxidant. Aromatic amines are metabolized and excreted through a process involving acetylation by N-acetyltransferase.8 Genetic variation in one of the genes, NAT2, encoding this enzyme produces either rapid or slow metabolic phenotypes in humans. Analysis of the NAT2 phenotypes of patients with bladder cancer from the dye industry indicates that persons showing the slow phenotype could be more susceptible to bladder cancer caused by aromatic amines. This finding is consistent with a meta-analysis indicating that NAT2 slow acetylation status is associated with an increased risk of bladder cancer in the general population.9 Exposure to benzene was suspected to be the cause of leukemia in a number of individual cases and case series reported worldwide between 1928 and 1976.1 Case-control studies indicated increased risks of nonlymphocytic leukemia among workers in Sweden exposed to petroleum products containing benzene and of lymphomas among workers in New York State who were exposed to benzene. Prospective studies conducted in the rubber industry provide the most convincing evidence for an association between benzene exposure and leukemia. Most of the excess leukemia in this industry is found among rubber workers exposed to solvents, including benzene. Excess mortality from leukemia has been observed among former employees of a rubber film production plant (O/E = 4.7) and a rubber coating plant (O/E = 3.7). The hepatotoxic effect of aflatoxins was first recognized when aflatoxin-contaminated feed was inadvertently fed to poultry. Subsequent animal studies demonstrated the carcinogenic potential of the aflatoxins, particularly aflatoxin B1. The aflatoxins are produced by the fungal strains Aspergillus flavus and A. parasiticus. Grains and foodstuffs for human consumption such as corn, peanuts, and rice can become contaminated with aflatoxin during growth or storage. The considerable variation in levels of human exposure to aflatoxin worldwide is determined by climate and by the preventive measures used to protect susceptible foods from mold contamination and growth.10 Dietary aflatoxin is correlated with high liver cancer rates in sub-Saharan Africa and Asia. Case-control studies in the Philippines and Mozambique show an increased risk of liver cancer with estimated levels of aflatoxin consumption. The co-carcinogenic role of hepatitis B virus (HBV) infection and dietary aflatoxin in liver cancer has been the focus of several studies. The incidence of liver cancer in different regions of Swaziland correlated more closely with aflatoxin intake than with HBV infection. A prospective study conducted in Guangxi Province, China, compared the incidence of liver cancer in regions of high and low aflatoxin contamination and determined HBV infection status.10 A strong interaction between aflatoxin exposure and HBV-positive status was observed for relative risk of liver cancer. Among HBV-positive individuals, the incidence of liver cancer was 649 per 100,000 in the high-aflatoxin region and 66 per 100,000 in the low-aflatoxin region, whereas among HBV-negative individuals, the incidence of liver cancer was 99 per 100,000 and <1 per 100,000 in high- or low-aflatoxin regions, respectively. The new techniques of molecular dosimetry for human carcinogen exposure have been applied in populations exposed to aflatoxin. With individual exposures often in excess of 10 to 100 µg/day, the presence of aflatoxin metabolites and DNA adducts can be quantified in the urine after exposure. The association of urinary aflatoxin-DNA adducts with risk of liver cancer also has been demonstrated in a prospective epidemiological study.11 Tobacco use causes more cancer deaths worldwide than any other human activity. Cigarette smoking is associated with cancers of the lung, oral cavity, pharynx, larynx, esophagus, bladder, renal pelvis, and pancreas (Box 9-1). The use of smokeless tobacco (chewing tobacco or snuff) leads to cancer of the oral cavity. Thus although combustion enhances the carcinogenic properties of tobacco, it is not required for cancer induction. Although the carcinogenic properties of tobacco tar were first demonstrated experimentally during the 1920s, evidence of a human cancer risk from the use of tobacco did not appear until 1939, when Muller and colleagues12
Environmental Factors
Introduction
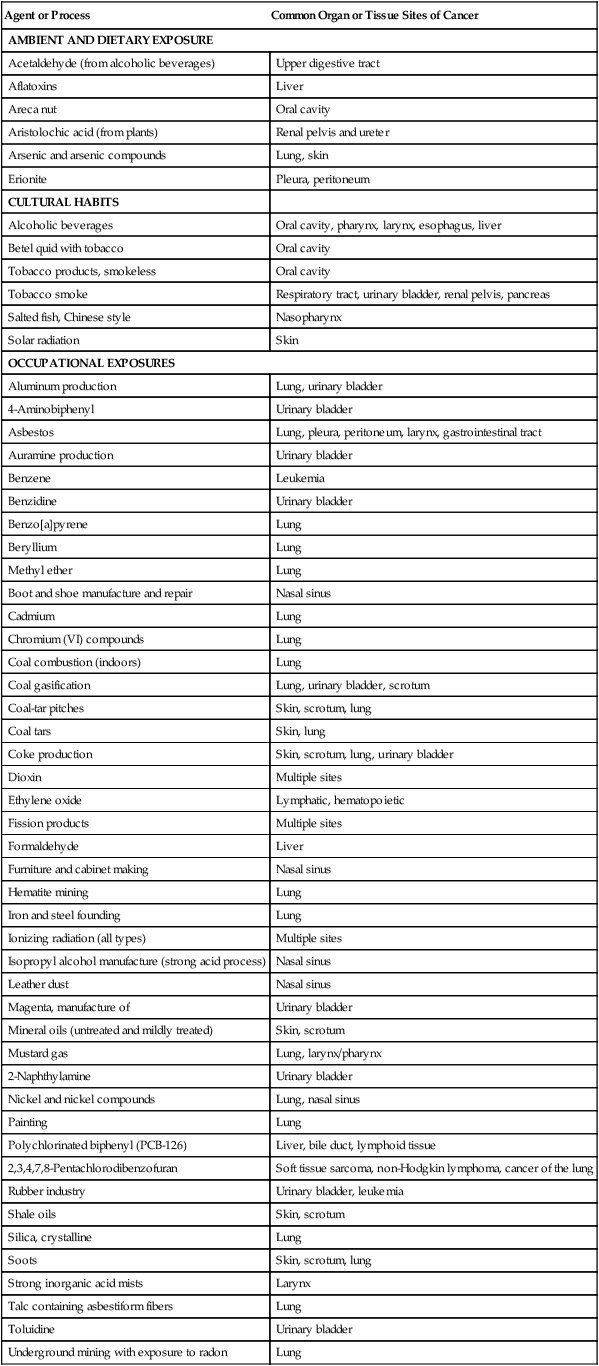
Agent or Process
Common Organ or Tissue Sites of Cancer
AMBIENT AND DIETARY EXPOSURE
Acetaldehyde (from alcoholic beverages)
Upper digestive tract
Aflatoxins
Liver
Areca nut
Oral cavity
Aristolochic acid (from plants)
Renal pelvis and ureter
Arsenic and arsenic compounds
Lung, skin
Erionite
Pleura, peritoneum
CULTURAL HABITS
Alcoholic beverages
Oral cavity, pharynx, larynx, esophagus, liver
Betel quid with tobacco
Oral cavity
Tobacco products, smokeless
Oral cavity
Tobacco smoke
Respiratory tract, urinary bladder, renal pelvis, pancreas
Salted fish, Chinese style
Nasopharynx
Solar radiation
Skin
OCCUPATIONAL EXPOSURES
Aluminum production
Lung, urinary bladder
4-Aminobiphenyl
Urinary bladder
Asbestos
Lung, pleura, peritoneum, larynx, gastrointestinal tract
Auramine production
Urinary bladder
Benzene
Leukemia
Benzidine
Urinary bladder
Benzo[a]pyrene
Lung
Beryllium
Lung
Methyl ether
Lung
Boot and shoe manufacture and repair
Nasal sinus
Cadmium
Lung
Chromium (VI) compounds
Lung
Coal combustion (indoors)
Lung
Coal gasification
Lung, urinary bladder, scrotum
Coal-tar pitches
Skin, scrotum, lung
Coal tars
Skin, lung
Coke production
Skin, scrotum, lung, urinary bladder
Dioxin
Multiple sites
Ethylene oxide
Lymphatic, hematopoietic
Fission products
Multiple sites
Formaldehyde
Liver
Furniture and cabinet making
Nasal sinus
Hematite mining
Lung
Iron and steel founding
Lung
Ionizing radiation (all types)
Multiple sites
Isopropyl alcohol manufacture (strong acid process)
Nasal sinus
Leather dust
Nasal sinus
Magenta, manufacture of
Urinary bladder
Mineral oils (untreated and mildly treated)
Skin, scrotum
Mustard gas
Lung, larynx/pharynx
2-Naphthylamine
Urinary bladder
Nickel and nickel compounds
Lung, nasal sinus
Painting
Lung
Polychlorinated biphenyl (PCB-126)
Liver, bile duct, lymphoid tissue
2,3,4,7,8-Pentachlorodibenzofuran
Soft tissue sarcoma, non-Hodgkin lymphoma, cancer of the lung
Rubber industry
Urinary bladder, leukemia
Shale oils
Skin, scrotum
Silica, crystalline
Lung
Soots
Skin, scrotum, lung
Strong inorganic acid mists
Larynx
Talc containing asbestiform fibers
Lung
Toluidine
Urinary bladder
Underground mining with exposure to radon
Lung
Vinyl chloride
Liver, lung, gastrointestinal tract, brain
Wood dust
Nasal cavities, paranasal sinuses
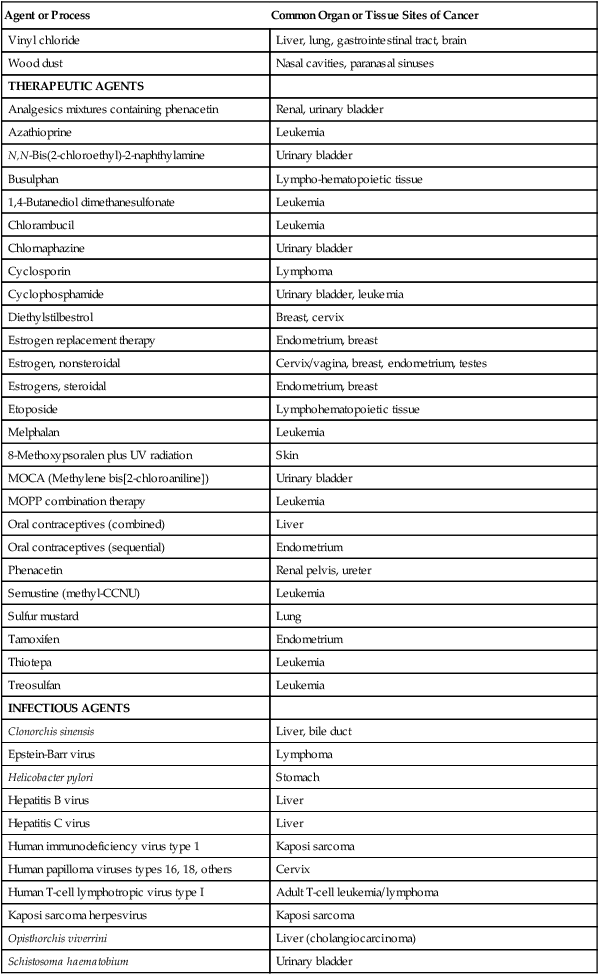
THERAPEUTIC AGENTS
Analgesics mixtures containing phenacetin
Renal, urinary bladder
Azathioprine
Leukemia
N,N-Bis(2-chloroethyl)-2-naphthylamine
Urinary bladder
Busulphan
Lympho-hematopoietic tissue
1,4-Butanediol dimethanesulfonate
Leukemia
Chlorambucil
Leukemia
Chlornaphazine
Urinary bladder
Cyclosporin
Lymphoma
Cyclophosphamide
Urinary bladder, leukemia
Diethylstilbestrol
Breast, cervix
Estrogen replacement therapy
Endometrium, breast
Estrogen, nonsteroidal
Cervix/vagina, breast, endometrium, testes
Estrogens, steroidal
Endometrium, breast
Etoposide
Lymphohematopoietic tissue
Melphalan
Leukemia
8-Methoxypsoralen plus UV radiation
Skin
MOCA (Methylene bis[2-chloroaniline])
Urinary bladder
MOPP combination therapy
Leukemia
Oral contraceptives (combined)
Liver
Oral contraceptives (sequential)
Endometrium
Phenacetin
Renal pelvis, ureter
Semustine (methyl-CCNU)
Leukemia
Sulfur mustard
Lung
Tamoxifen
Endometrium
Thiotepa
Leukemia
Treosulfan
Leukemia
INFECTIOUS AGENTS
Clonorchis sinensis
Liver, bile duct
Epstein-Barr virus
Lymphoma
Helicobacter pylori
Stomach
Hepatitis B virus
Liver
Hepatitis C virus
Liver
Human immunodeficiency virus type 1
Kaposi sarcoma
Human papilloma viruses types 16, 18, others
Cervix
Human T-cell lymphotropic virus type I
Adult T-cell leukemia/lymphoma
Kaposi sarcoma herpesvirus
Kaposi sarcoma
Opisthorchis viverrini
Liver (cholangiocarcinoma)
Schistosoma haematobium
Urinary bladder
Role of Environmental Agents in the Etiology of Human Cancers
Chemicals
Polycyclic Aromatic Hydrocarbons
Aromatic Amines
Benzene
Aflatoxins
Tobacco Chemicals
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Environmental Factors