Enteral Nutrition Support
Vanderbilt University Medical Center, Nashville, TN
Tube feeding, or enteral nutrition, is a method of providing nutrition support to patients with a functional gastrointestinal (GI) tract who are unable to ingest adequate nutrients by mouth. Tube feeding provides many physiologic, metabolic, safety, and cost advantages over parenteral nutrition and should be utilized when possible. Since there are many tube feeding formulas available on the market, understanding formula composition is important when selecting tube feeds for an individual patient. Assessment of the patient’s nutrient requirements, GI anatomy and function, and length of time for nutrition support, and clinical condition should be evaluated prior to selecting feeding tube access and mode of administration. Once the decision is made to start tube feeds, monitoring is crucial to prevent complications.
Tube Feeding Indications
A nutrition assessment is a helpful tool in determining which patients may benefit from tube feeds. Hospitalized patients may require short-term tube feeding if they are unable to eat due to mental status changes, poor appetite, dysphagia, or mechanical ventilation. Tube feeding in the home setting may be appropriate for patients with ongoing difficulty eating. Advances in tube feeding have made enteral nutrition possible in conditions such as acute pancreatitis, high-output enterocutaneous fistulae, and open abdomen. Tube feeding would not be initiated if a patient has diffuse peritonitis, intestinal obstruction, intractable vomiting or diarrhea, paralytic ileus, or GI ischemia. Although it is occasionally used in patients with short bowel syndrome and severe malabsorption, it should not be relied upon as the primary means of nutrition in sick or malnourished patients. In situations where a patient or caregiver is refusing tube feeds, it is important for a clinician to assist with providing goals of care. Discussing the burden of tube feeds versus its benefits can help guide the decision for whether tube feeds should be provided for palliative care and hospice patients. Guidelines established by the American Society of Parenteral and Enteral Nutrition (ASPEN) recommend initiating nutrition support when patients are expected to (or have) not received adequate oral intake for 7 to 14 days. However, patients who are malnourished or stressed may require earlier initiation of nutrition support.
Tube Feeding Advantages
The old adage “if the gut works, use it” continues to be supported by clinical and experimental studies that compare enteral to parenteral nutrition. It is important to consider bowel length and the condition of the bowel when determining if the GI tract is functioning adequately. Tube feeding offers many potential advantages over parenteral nutrition, including lower rates of infectious and metabolic complications, decreased hospital length of stay, and reduced cost. Tube feeding is more physiologic than parenteral nutrition and promotes efficient utilization of substrates. Significant immune benefits when using tube feeds versus parenteral nutrition have also been reported in a variety of patient populations. Enteral stimulation plays an important role in the immune system. Gut-associated lymphoid tissue (GALT) produces antigens and helps prevent translocation of bacteria across the mucosal barrier. It has been proposed that the benefits of tube feeding are partially due to its ability to preserve gut integrity and GALT function. Studies in patients with burns, head injury, and trauma have shown decreased rates of infection in those receiving tube feeds compared to those given parenteral nutrition. Certain nutrients, such as fiber, intact proteins and peptides, and specialized fatty acids can be added to tube feeds but not parenteral nutrition. Tube feeds also stimulate the release of cholecystokinin, which maintains normal gallbladder function. For critical care patients, early post-operative tube feeding has been shown to reduce rates of infection, hospital length of stay, and trend toward reduced anastomotic dehiscence when compared to patients receiving no nutrition support therapy. These factors make tube feeds the preferred method for providing nutrition support.
Nutritional Content of Tube Feeding Formula
The type and quantity of protein, carbohydrate, and fat varies among tube feeding formulas. The amount of protein contained in a tube feeding formula can range from 8 to 25 percent of total calories. Nitrogen can be supplied as whole protein, partially hydrolyzed protein (peptides), or fully hydrolyzed protein (free amino acids). Individual amino acids, such as glutamine and arginine, have recently been added to some specialized formulas along with whole protein in order to promote immune function and improve bowel integrity.
Carbohydrate is the primary energy source in standard enteral formulas. The amount of carbohydrate in a tube feeding formula can range from 27 to 70 percent of total calories. Carbohydrate sources come from large molecules such as glucose oligosaccharides, maltodextrin, and hydrolyzed cornstarch. Smaller molecules such as mono- and disaccharides may be used and require a smaller amount of pancreatic enzymes and intestinal mucosal disaccharides for adequate digestion. Most commercially made tube feeding formulas are lactose and gluten free but some supplements designed for oral intake and infant formulas may contain lactose.
Fat is included as a component in tube feeding formulas to provide a concentrated energy source and essential fatty acids. The amount of fat in a tube feeding formula can range from 10 to 55 percent of total calories. Formulas typically contain a combination of long-chain triglycerides (LCT) and medium-chain triglycerides (MCT). LCT is more calorically dense than MCT (9 kcal/g versus 8 kcal/g) and is the only source of essential omega-6 polyunsaturated fatty acids. Omega-3 polyunsaturated fatty acids have been added to some specialized tube feeding formulas for their anti-inflammatory properties. LCT fats require an intact digestive tract for maximal absorption. Sources of LCT are primarily soybean and corn oil; however, safflower, canola, and fish oils are also used. MCT is easier than LCT to process as they are more miscible in water, are more readily hydrolyzed by pancreatic enzymes, do not require chylomicron formation, are transported directly to the liver through portal circulation, and are rapidly metabolized as fuel when they reach the liver. Sources of MCT include palm kernel and coconut oil.
Some tube feeding formulas contain added fiber (4 to 20 g/L), usually as a mixture of both soluble and insoluble fibers. Fiber helps normalize bowel function by increasing stool bulk and by indirectly providing energy for colonocytes. Soluble fibers are metabolized by the intestinal flora of the colon which produce the short-chain fatty acids acetate, proprionate, and butyrate. Butyrate is the primary fuel of the colonocyte and as a result can improve absorptive function of the colon and lead to restitution of injured and inflamed mucosa. Short chain fatty acids lower the intraluminal pH of the colon and promote the growth of intestinal flora which helps protect against pathogenic strains of bacteria. Dietary fibers that lead to a favorable change of the intestinal flora are referred to as prebiotics. Fructooligosaccharides (FOS) and inulin are the soluble fibers that are added to tube feeding products because of their prebiotic effects. Fiber does however increase the viscosity of formulas and can contribute to the clogging of feeding tubes. As a result, it is best to administer fiber-containing formulas through a larger tube (≥8 ft) to avoid tube clogging.
Vitamins, minerals, and trace elements are also included in standard tube feeding formulas. Most formulas meet the United States Dietary Reference Intake (DRI) for these nutrients in 1.0 to 1.5 liters of formula. A vitamin and mineral supplement is appropriate for patients receiving less than the necessary volume to meet the requirements. Antioxidant vitamins (vitamin C and E) have been added to formulas to support specific diseases associated with oxidative stress such as inflammatory bowel disease and acute respiratory distress syndrome (ARDS). Tube feeding formulas also contain 70 to 85 percent water. In general, tube feeds are not meant to provide full hydration for a patient and additional fluid should be given as water flushes through the feeding tube or by the intravenous route.
Formula Selection
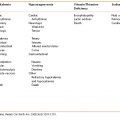
There are four main categories of tube feeding formulas; standard or polymeric, elemental and semi-elemental, disease specific, and immune-enhancing. Standard formulas are isotonic and contain intact protein, complex carbohydrates, and a higher amount of LCT than MCT. These formulas are typically used for patients with adequate digestion and absorption ability. Elemental and semi-elemental formulas contain hydrolyzed protein, either as free amino acid or peptides, and have a higher tonicity than standard formulas due to the predigested protein source. Elemental and semi-elemental formulas are intended for patients with impaired digestion or absorption, such as those with Crohn’s disease, pancreatic insufficiency, radiation enteritis, and short bowel syndrome. Disease-specific formulas were developed for patients with glucose intolerance and kidney, liver, and pulmonary disease. They have a varying amount of protein, carbohydrate, fat, and electrolytes based on the needs of the specific disease state. Immune-enhancing formulas have been shown to improve immune cell function, modulate inflammation, and reduce infection in specific patient populations. They contain the addition of varying amounts of glutamine, arginine, nucleotides, omega-3 fatty acids, and antioxidants. Benefits of immune-enhancing formulas have been shown in the following patient population: critically ill patients on mechanical ventilation, major elective surgery, trauma, burns, and head and neck cancer (Table 12-1).
Table 12-1 Classification of Tube Feeding Formulas
| Formula Type | Characteristics |
|---|---|
| Polymeric/standard | Whole protein, polysaccharide, and mixture of fat sources |
| Nutrient dense | Polymeric with reduced water (60–70%) |
| High nitrogen | Over 20% of calories as protein |
| Elemental/semi-elemental | Oligopeptides and free amino acids (elemental) in place of whole proteins, low-fat, and/or higher concentration of MCT* |
| Immune-enhancing | Added glutamine, arginine, omega-3, and antioxidants |
| Hepatic disease | Increased branch chain amino acids and reduced aromatic amino acids |
| Renal disease | Reduced protein, water, electrolytes and minerals. May contain few non-essential amino acids |
| Glucose intolerance | High-fat, low-carbohydrate, fiber |
| Pulmonary disease | High-fat, low-carbohydrate, omega-3 fatty acids, and antioxidants |
*MCT = medium chain triglycerides.
There are many disease-specific formulas available on the market. Products designed for hepatic encephalopathy have a very specific branch chain to aromatic amino acid ratio. Use of these formulas should be limited to patients with advanced liver disease and hepatic encephalopathy who fail to respond to conventional therapy. Formulas designed for patients with renal failure restrict fluids, potassium, phosphorus, and magnesium to match the dietary restrictions of this population. These products should only be used in renal disease when dialysis is inadequate. Specialty formulas designed to improve glucose control contain a lower concentration of carbohydrate and are supplemented with fiber. These formulas are beneficial for patients who have uncontrolled blood glucose levels when using a standard polymeric formula in addition to oral hypoglycemic medication or insulin. They should not be routinely used for all diabetic patients as they tend to be higher in fat and are more expensive. Formulas designed for respiratory insufficiency contain 40 to 50 percent of total calories as fat to minimize the production of carbon dioxide through carbohydrate metabolism. These formulas are also nutrient dense for fluid restriction in patients with pulmonary edema. Use of these formulas should be limited to patients that continue to have respiratory difficulty in spite of standard treatments. For patients with acute lung injury or ARDS, an enteral formula supplemented with eicosapentaenoic acid (EPA), gamma-linolenic acid (GLA), and antioxidants is available with the aim of down-regulating the inflammatory response. A recent meta-analysis, including three randomized controlled studies, revealed a significant reduction in the risk of mortality and improved outcomes in patients receiving tube feeds containing EPA and GLA.
Immune-enhancing formulas have been shown to be beneficial in lowering infection rates, reducing infectious complications, and shortening length of stay when used in certain population groups. Glutamine is supplemented in formulas to promote immune function and improve bowel integrity. Under appropriate experimental conditions glutamine has been shown to be essential for cell proliferation, it can act as a respiratory fuel, and it can enhance the function of stimulated immune cells. Patients that seem to benefit most from glutamine-containing formulas are those with post-operative wound infections, cancer requiring surgery, massive blood infusions, trauma, and acutely ill immune-suppressed patients. Arginine has also been supplemented in some formulas to prevent muscle wasting and promote wound healing and immune function. The benefits of arginine have been best demonstrated in surgical patients. The benefit in critically ill patients is not clear.
Calorically dense formulas (1.5 to 2.0 kcal/mL) are available for patients requiring fluid restriction. These formulas provide less water and have a modestly higher viscosity and osmolality compared to standard formulas. Occasionally, nutrient-dense formulas are used to decrease infusion time or the daily volume of tube feeding. If this is done for a patient that is not fluid restricted then additional water may need to be given to avoid dehydration.
When selecting a tube feeding formula it is important to consider a patient’s nutrient requirements, GI function, and overall clinical condition. Standard polymeric formulas should be used unless there is a clear indication that a patient would benefit from a specialty formula. Standard formulas are less expensive than specialty products and should be the formula of choice for many patient populations.
Selecting the Feeding Route
When selecting a route for tube feeding, it is important to consider estimated length of need, GI anatomy and function, and clinical condition. Short-term therapy, defined as 4 to 6 weeks, is typically managed with a nasogastric or nasoenteric tube. Nasoenteric tubes most frequently terminate within the duodenum. These can be placed at the bedside blindly or with an electromagnetic tube placement device. Radiologic or endoscopic techniques can also be used when the aforementioned methods are not successful; the patient requires positioning of the tube from the stomach into the small bowel, or when initial jejunal placement is necessary. Placement generally imposes little risk to patients except when tubes are placed blindly they may be inadvertently placed into the lungs. This risk is greatest for patients with an altered mental status or gag reflex but can also occur in patients without these problems. Radiographic confirmation is therefore highly recommended when tubes are placed or repositioned blindly. An advantage of nasogastric and nasoenteric tubes versus percutaneous tubes, which are discussed next, is that the tubes can be easily removed when tube feeding is no longer needed. Orogastric and oroenteric tubes are occasionally used for short-term therapy with the main indications being facial or nasal injury, nasal deformity that precludes nasal tube placement, and the presence of sinusitis.
Patients who require long-term tube feeding often prefer a percutaneous feeding tube that is less visible and more comfortable than a nasal or oral tube. Insertion options for percutaneous feeding tubes include endoscopic, radiologic, and surgical (laparoscopic or open) techniques. Recently, it has been suggested that the terms transoral or transabdominal access be used to further describe how the feeding tube is brought into position with either the endoscopic and radiologic techniques. All three techniques can be used to place a tube into the stomach that is generally referred to as a gastrostomy tube. When it is placed it may be referred to as a percutaneous endoscopic gastrostomy (PEG). Tubes that are placed into the stomach with a portion of the tube being advanced through the pylorus into the proximal small bowel are used to provide feeding into the small bowel and simultaneous decompression of the stomach. They can be placed using all three techniques and are referred to as gastrojejunostomy tubes. Percutaneous feeding tubes placed directly into the jejunum, or jejunostomy tubes, are most often placed using an endoscopic or surgical approach as radiographic placement is technically challenging. These tubes are preferred when gastric decompression is not needed or when the end of a gastrojejunostomy tube cannot be maintained in the small bowel. Percutaneous feeding tube placement carries some risks that include bleeding, wound infection, bowel perforation, obstruction, and the risks of anesthesia. However, these risks are minimized when an experienced physician is performing the procedure and the patient’s clinical and nutritional status is not severely compromised.
Feeding into the stomach is the most physiologic way to deliver tube feeding as it allows feeding to be administered intermittently. It also requires the least amount of equipment to administer feeding which makes this approach less costly and more convenient than feeding into the small bowel. Gastric feeding tubes are used in the majority of patients who receive home tube feeding. Small bowel feeding tubes, which are ideally placed past the ligament of Treitz, are used for patients with delayed gastric emptying or gastric outlet obstruction. Additional conditions where this approach might be favored include patients with tracheal aspiration, reflux esophagitis, previous gastric surgery, early post-operative feeding, and where gastric feeding is poorly tolerated. Feeding beyond the ligament of Treitz has been shown to minimize stimulation of the exocrine pancreas and is currently considered the favored route for providing nutrition support to patients with severe acute pancreatitis. Although controversial, many clinicians feel that small bowel tube feeding may decrease the risk of aspiration and will therefore use this approach to minimize the risk of aspiration pneumonia.
Administering Tube Feeding
Tube feeding schedules should be designed around the patient’s clinical condition, physical activity, and the feeding access. Tube feeding can be administered as a bolus, as a gravity drip, or continuously for an extended length of time. Bolus feedings are defined as formula delivered via a syringe over approximately 15 minutes. Gravity drip feedings, sometimes referred to as intermittent feedings, are delivered using tubing outfitted with a roller clamp to control the rate of infusion. Gravity drip feeds are typically infused over 30 to 45 minutes. These two feeding techniques should only be administered via a gastric feeding tube because of the stomach’s ability to accommodate a large volume. In general, bolus feeding is preferred for patients with gastrostomy tubes in the home setting as it allows them to follow a meal pattern, requires a minimal amount of equipment, and allows the greatest degree of flexibility while managing other routines of daily living.
Small bowel tube feeding should be administered with a feeding pump to allow prolonged constant infusion to minimize feeding intolerance. Feeding by this method is typically done over 8 to 24 hours. Patients who are critically ill often have abnormal GI motility and may require feeding over 24 hours to receive their entire tube feeding prescription, while patients who are less ill and often more mobile are good candidates for tube feeding that is infused more quickly over a shorter length of time. While the continuous feeding method is always done for patients with jejunostomy feeding tubes, this technique may also be used for patients with gastrostomy tubes who do not tolerate bolus or intermittent feedings. In patients who are being transitioned to oral intake, tube feeding is sometimes administered continuously overnight to limit appetite suppression that can occur when eating and tube feeding is done simultaneously.
In order to allow for GI adaptation to tube feeding, continuous tube feeding is generally initiated at 10 to 30 mL/h. The feeding volume is then increased by increments of 10 to 20 mL/h every 6 to 12 hours, depending on tolerance. Intermittent or bolus feedings are usually initiated at 60 to 120 mL and then advanced as tolerated to the goal volume. Mild bloating and loose bowel movements are common when tube feeding is initiated. If the patient shows any signs of severe intolerance to the feeding, such as diarrhea, elevated gastric residuals, or vomiting, the administration should not be sustained and may be discontinued temporarily while the patient undergoes appropriate clinical evaluation.
Monitoring
Once tube feeds are initiated, monitoring is important to ensure that adequate nutrients are provided and to help prevent complications or manage them soon after they arise. A combination of physical assessment, laboratory data, and assessing GI function are used when determining patients’ tolerance to tube feeds.
Feedings received should be compared to prescribed calorie and protein goals. Adjustments in prescriptions should be made for changes in clinical status or activity. Calories and carbohydrate provided from tube feeding and other sources such as intravenous fluids should be routinely monitored and adjusted to avoid the complications due to overfeeding. In critically ill patients, especially those with underlying pulmonary disease or recent injury, excessive carbon dioxide production caused by overfeeding can cause difficulty in ventilator support and weaning. If a patient is not responding appropriately to the tube feeding prescription, indirect calorimetry can be used to better define energy needs. In order to monitor hydration, it is important to evaluate fluid intake/output and daily weights, especially in hospitalized patients. A rapid change in weight may suggest an alteration in hydration and should prompt further investigation. It is often helpful to establish a target weight for patients who require tube feeding outside of the hospital to ensure that they are receiving an appropriate calorie prescription. In many instances, patients who have been ill and lost weight need to achieve their pre-illness weight to fully recover. In patients who are overweight or obese, it may be desirable to promote gradual weight reduction for its many health benefits. Weight loss can be achieved by targeting energy intake modestly below requirements while providing a sufficient amount of nitrogen to promote wound healing and hepatic protein production. The patient’s strength and feeling of wellbeing is another important indicator of adequate nutrient delivery. Finally, continued need for nutrition support should be routinely reevaluated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree