Indications (low risk)
High risk
Unifocal (limited or focal), flat HGD
Multifocal HGD, HGD with nodules
Type I, IIa < 2 cm, IIb, IIc < 1 cm
Type I, II > 3 cm, type III
Well- or moderately differentiated adenocarcinoma
Poorly differentiated adenocarcinoma
Lesions limited to the mucosa (m)
Invasion into the submucosa (sm)
No lymphovascular invasion
Presence of lymphovascular invasion
Esophageal squamous cell cancer appears to be biologically more aggressive than adenocarcinoma, and the risk of lymph node involvement is higher in patients with squamous cell cancer. Patients with intraepithelial cancers (m1) and cancers invading the lamina propria (m2) have almost no risk of lymph node involvement [21– 23], whereas the risk of lymph node involvement in cancers invading the muscularis mucosa (m3) and the submucosa (sm) ranges from 0 to 10 % [23] and from 50 to 55 % [22], respectively. For patients with esophageal squamous cell cancer, esophageal-preserving therapy can be indicated only for superficial (m1 and m2) cancers with well-to-moderate differentiation and no lymphovascular invasion. Patients with m3 cancers could be candidates for esophageal-preserving therapy if there are no further risk factors for lymph node involvement. Risk factors for esophageal squamous cell cancer are summarized in Table 2.2.
Table 2.2
Indications for endoscopic resection of esophageal squamous cell carcinoma (SCC)
Indications (low risk) | High risk |
|---|---|
No consensus on the maximal size | |
Well- or moderately differentiated SCC | Poorly differentiated SCC |
Limited to the lamina propria (m1–2) | Invasion into the deeper layer than the muscularis mucosa (m3, sm) |
No lymphovascular invasion | Presence of lymphovascular invasion |
T1b Esophageal Cancer
Once tumors invade the submucosal layer, the probability of lymph node involvement is exponentially increased due to the abundant submucosal lymphatic networks [24, 25]. Therefore, esophagectomy has been recommended as a standard of care for patients with T1b esophageal cancer. A recent review using the pooled data of 7,645 patients with T1b submucosal esophageal cancer has demonstrated that the overall rate of lymph node involvement in T1b cancers was 37 %; however, there was a substantial difference between T1sm1 and T1sm2/3 adenocarcinoma (6 % vs. 23 %/58 %, respectively), suggesting that highly selected patients with T1sm1 adenocarcinoma could be candidates for esophageal-preserving therapy [26]. This is further supported by the most recent study involving 66 patients with low-risk T1sm1 cancer (macroscopically polypoid or flat lesion, well-to-moderate differentiation and no lymphovascular invasion) demonstrating that complete remission was achieved in 97 % of patients with small nodules ≤2 cm, and long-term remission without any metachronous disease was achieved in 90 %. There were no tumor-related deaths and the estimated 5-year survival was 84 %, although one patient developed lymph node metastasis [27]. The risk of developing lymph node metastasis after esophageal-preserving therapy for T1sm1 appears to be lower than the mortality rate of esophagectomy, suggesting that patients with low-risk T1sm1 adenocarcinoma could be a candidate for esophageal-preserving therapy, particularly when poor functional status and comorbid conditions make esophagectomy too risky. T1sm2 and sm3 adenocarcinoma and all T1b squamous cell carcinomas are associated with a high rate of lymph node involvement, and esophagectomy should be considered [28, 29]. It is noted that these data may not be transferable to patients at all centers delivering therapy because these data were achieved within high-volume, experienced centers.
Clinical Staging
Accurate clinical staging is essential for esophageal-preserving therapy and it is extremely important to exclude patients with potential lymph node involvement and/or metastatic disease. Therefore, all patients require positron-emission tomography/computed tomography (PET/CT), endoscopic ultrasound (EUS), and diagnostic endoscopic mucosal resection (EMR) for staging purposes, when esophageal-preserving therapy is considered. Since approximately 25 % of all patients with esophageal cancer have metastatic disease identified by PET/CT and this yield is far superior to the combination of EUS and CT scan [30, 31], PET/CT has been utilized to assess metastatic disease.
EUS has been utilized to assess tumor depth (T-stage) and lymph node involvement (N-stage). A recent meta-analysis has demonstrated that the pooled sensitivity and specificity of EUS to diagnose T1 stage cancer was 81.6 and 99.4 %, respectively [32], suggesting that EUS cannot accurately differentiate T1a from T1b esophageal cancers. To improve the diagnostic yield of T1 stage tumors, a high-frequency EUS miniprobe (20 or 30 MHz) has been introduced and investigated. A recent retrospective study demonstrated that the overall accuracy, sensitivity, and specificity to differentiate T1b from T1a cancers with high-frequency miniprobes were 73.5, 62, and 76.5 %, respectively, suggesting that even the high-frequency miniprobe still has a limited accuracy for the diagnosis of T1a cancer [33]. Other meta-analysis has demonstrated that pooled sensitivity and specificity of EUS for regional lymph node involvement were 76.4 and 72.4 %, respectively, suggesting EUS is also not satisfactory for the assessment of N-staging [34]. It is important to understand the limitation of EUS in the staging process.
Since EUS is not reliable for T- and N-staging, a diagnostic EMR for the staging purpose is essential to exclude any possibility of submucosal (T1b) or deeper invasion (>T2) and predict potential lymph node involvement based on complete histological assessment. EMR provides complete specimens including both mucosa and submucosa for histological assessment of lateral and deep margins, thereby determining the accurate T-stage (i.e., differentiating T1a from T1b). A positive lateral margin can be addressed with additional endoscopic intervention, while a positive deep margin should be considered for esophagectomy.
Endoscopic Ablation
The purpose of endoscopic ablation therapy is to eradicate disease by ablating (burning or freezing) the affected epithelium of the esophagus. Currently, radiofrequency ablation (RFA) and cryotherapy have been primarily performed as endoscopic ablation therapy. The common drawback of ablation therapy is that there is no specimen available for histological assessment.
Radiofrequency Ablation (RFA)
RFA using the Barrx™ Ablation System (Covidien, Sunnyvale, CA) has been most commonly used as ablation therapy, since the multicenter, randomized, sham-controlled trial involving 127 patients with Barrett’s esophagus demonstrated that 81 % of patients with HGD had complete eradication of dysplasia with RFA compared to 19 % in the control group (no RFA) (p <0.001) and patients who underwent RFA had significantly less disease progression and fewer cancers developed during the follow-up of 12 months [35]. Either an ablation balloon catheter (Barrx™ 360 RFA Balloon Catheter) for circumferential ablation or an endoscopic mounted device (Barrx™ 90, 60, Ultra Long RFA Focal Catheter) for focal ablation can be selected based on the length, extension, and location of disease (Fig. 2.1). This system delivers a high-power, ultrashort burst of ablative energy to the abnormal esophageal epithelium, and the delivered energy provides uniform treatment to a depth of approximately 500 μm. Therefore, the depth of treatment is limited to the mucosal layer, thereby significantly reducing the risk of stricture formation. The rate of stricture formation was reported to be 6 % [35]. A further follow-up study demonstrated that patients’ quality of life significantly improved after the RFA treatment, although most patients were worried about esophageal cancer and esophagectomy before the RFA treatment [36]. Due to the limited depth of treatment, RFA is not indicated for invasive cancer.
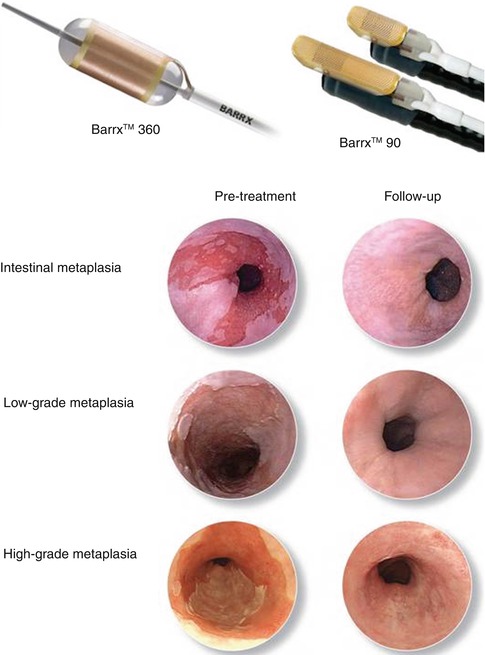

Fig. 2.1
Radiofrequency ablation therapy. The upper panels show the Barrx™ (left) and Barrx™ systems (right). The lower panel shows endoscopic findings of pre- and post-treatment for intestinal metaplasia (top), low-grade dysplasia (middle), and high-grade dysplasia (bottom) (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)
Cryotherapy
Cryotherapy involves the topical application by spraying aerosolized liquid nitrogen or carbon dioxide onto the abnormal esophageal epithelium, providing intracellular disruption and ischemia while preserving the extracellular matrix and thereby minimizing fibrosis. A prospective study involving 98 patients with HGD has demonstrated that 97 % had complete eradication of HGD with no esophageal perforation [28]. Current cryotherapy devices require a venting system such as a nasogastric tube to help excessive nitrogen gas escape out of the esophagus and stomach, thus preventing perforation of the gastrointestinal tract. Furthermore, cryotherapy is associated with several issues including its nonuniform application using a handheld catheter, the fogging of scope lens, and the prolonged duration of the therapy. Currently, a novel through-the-scope cryoballoon device, which does not require a venting system and potentially delivers a uniform and reproducible ablation, has been under investigation, and further study to evaluate the safety and efficacy of this device is awaited.
Endoscopic Resection
The goal of endoscopic resection is to completely remove the entire segment of abnormal esophageal epithelium, thereby curing HGD and T1a cancers. Unlike endoscopic ablation therapy, endoscopic resection can provide specimens for the complete histological assessment including depth of cancer invasion, degree of cellular differentiation, and involvement of lymphatics or vessels. Currently, endoscopic mucosal resection (EMR) is used for the lesions less than 2 cm, and endoscopic submucosal dissection (ESD) is recommended for en bloc removal of lesions larger than 2 cm. The major complication associated with endoscopic resection is stricture formation, especially when more than 75 % of the esophageal circumference is involved in a single setting [29]. Small clinical series have reported that the stricture rate after circumferential EMR is up to 80 % [37, 38].
Endoscopic Mucosal Resection (EMR)
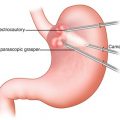
EMR has been commonly used as both a diagnostic and therapeutic tool. There are primarily two techniques: cap resection technique and ligate-and-cut technique (Fig. 2.2) [39]. A randomized trial comparing these two techniques has demonstrated no difference in safety and efficacy between the techniques [40]. Both techniques are initiated by injecting normal saline into the submucosal space to lift the lesions away from the muscularis propria. The injection needle should be inserted into a submucosal space at the sharp angle to avoid transmural penetration of the needle. Injected saline acts as a “safety cushion” between the mucosa and muscularis propria to reduce the risk of unexpected complications such as perforation during the procedure. Additional injection of saline is sometimes required because the injected saline disappears within a few minutes. Difficulty lifting up the lesion by submucosal injection suggests invasion into the muscularis propria. For the cap resection technique, a clear plastic cap (either straight or oblique shaped) is attached to the tip of endoscope. The oblique-shaped caps are usually used for esophageal lesions, while the straight caps are most commonly used for the lesions in the stomach and colon. The mucosal-submucosal complex is then sucked into a cap mounted on an endoscope, creating a pseudopolyp. The pseudopolyp is then resected by being captured at its base with a cautery snare which is positioned inside the cap [41]. For the ligate-and-cut technique, the only difference to the cap technique is to deploy a band to create a pseudopolyp [26, 27]. Currently, there is a novel multiband mucosectomy device available, which uses a specially designed multiple-band ligator and allows endoscopists to perform ligation and subsequent immediate resection without removal of the endoscope by passing a cautery snare through the ligator handle. The retrieved specimen is pinned to a piece of cork and placed into preservative solution prior to processing for histological assessment. EMR is indicated for lesions less than 2 cm in diameter. Although en-bloc resection is ideal in any situation, piecemeal resection of large lesions (>2 cm) is acceptable; however, several studies have shown that piecemeal EMR is associated with incomplete resection and compromised histological assessment, likely causing the development of metachronous lesions [42, 43].
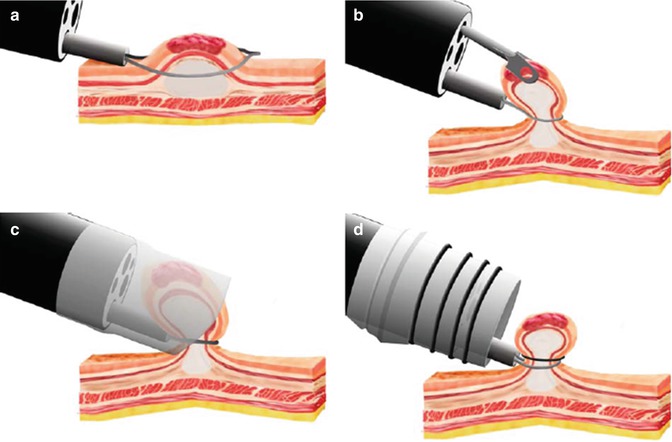

Fig. 2.2
Four types of endoscopic mucosal resection (EMR) techniques. (a) Snare polypectomy. (b) Strip biopsy technique. (c) The cap resection technique. (d) The ligate-and-cut technique (From Soetikno et al. [39] with permission)
An early retrospective study from a single institution demonstrated that 98 % of patients with HGD and T1a adenocarcinoma (n = 115) achieved complete response to EMR; however, 30 % of patients developed metachronous cancers during a mean follow-up of 34 months [42]. In a further study from the same institution, several factors including piecemeal resection, long-segment BE, no ablation therapy of BE after a complete response, multifocal neoplasia, and time until complete response >10 months were found to be associated with frequent tumor recurrence after endoscopic resection (Table 2.3) [43]. Based on these results, combination therapy involving focal EMR to resect nodules followed by RFA to treat any residual flat Barrett’s epithelium has been investigated to minimize the development of recurrent disease. A recent multicenter, prospective study to evaluate the efficacy of this combination therapy has demonstrated that 95 % of patients with HGD or T1a adenocarcinoma (n = 24) had a complete response to the combination therapy and no recurrence occurred during a median follow-up of 22 months [44]. These studies emphasize the importance of intensive surveillance, the risk of metachronous lesions after endoscopic resection, the need for post-intervention intensive surveillance, and the necessity of discussing the possibility of recurrent cancers with patients prior to the initiation of endoscopic interventions.
Table 2.3
Risk factors potentially associated with recurrence after endoscopic resection of early esophageal cancer
Risk factors for recurrence after endoscopic resection of early esophageal cancer |
|---|
1. Piecemeal resection |
2. Long-segment BE |
3. No ablation therapy of BE after CR |
4. Time until CR achieved > 10 months |
5. Multifocal neoplasia |
Endoscopic Submucosal Dissection (ESD)
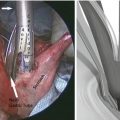
ESD has been established as an advanced endoscopic resection technique to accomplish en bloc resection of lesions larger than 2 cm in diameter. ESD is expected to provide more accurate histological assessment for the lateral and deep margins of lesions and thus prevent or minimize the development of metachronous lesions. ESD employs the same concept of EMR but requires some modifications for en bloc resection of a large lesion. Each step is summarized in Fig. 2.3. ESD is initiated by a mucosal marking around the lesion by using electrocautery, thus easily identifying the location of the entire lesion after the submucosal injection (Fig. 2.4a). Subsequently, a solution is injected into the submucosal space to lift the lesion away from the muscularis propria. The injection solutions for ESD include normal saline, glycerol, and sodium hyaluronate. Sodium hyaluronate stays longer in the submucosal space than normal saline or glycerol and may be ideal for ESD. Diluted sodium hyaluronate (approximately 0.5 % solution) is usually mixed with epinephrine (0.01 mg/ml) and indigo carmine (0.04 mg/ml). The mucosal cutting is then performed to create the entry to the submucosal space by using a specialized endoscopic electocautery called “needle knife” (Fig. 2.4b, c). Several types of needle knives having different shaped tips are available, depending on the preference of endoscopists (Fig. 2.5). Once the submucosal space is entered, tension and counter-tension are maintained by a cap mounted on the tip of endoscope, which is placed in the plane between the mucosal-submucosal complex and the muscularis propria. The needle knife is then introduced through the endoscopic working channel, and the attachments and bridging vessels between the two layers are dissected. At the completion of this procedure, the lesion can be resected en bloc regardless of its size and the remaining thin layer of sm3 can be seen over the resected area (Fig. 2.4d). It is important to maintain this thin layer of sm3, especially when repair of a perforation is required. ESD is a “one-person” procedure and does not allow for assistant hands. It is therefore important to maintain adequate counter-traction on the mucosa to be resected throughout the procedure. For this purpose, it may be better to start with a partial mucosal incision rather than a circumferential mucosal incision, maintaining the continuity of the lesion to the esophageal epithelium as “counter-traction,” and mucosal incision and submucosal dissection should be repeated step by step. The advantage of gravity should be considered; submucosal dissection should be started from the upper portion of the lesion so that the dissected mucosa is pulled down by gravity, spontaneously exposing the submucosal layer. It is also worth considering repositioning patients to obtain the advantage of gravity.
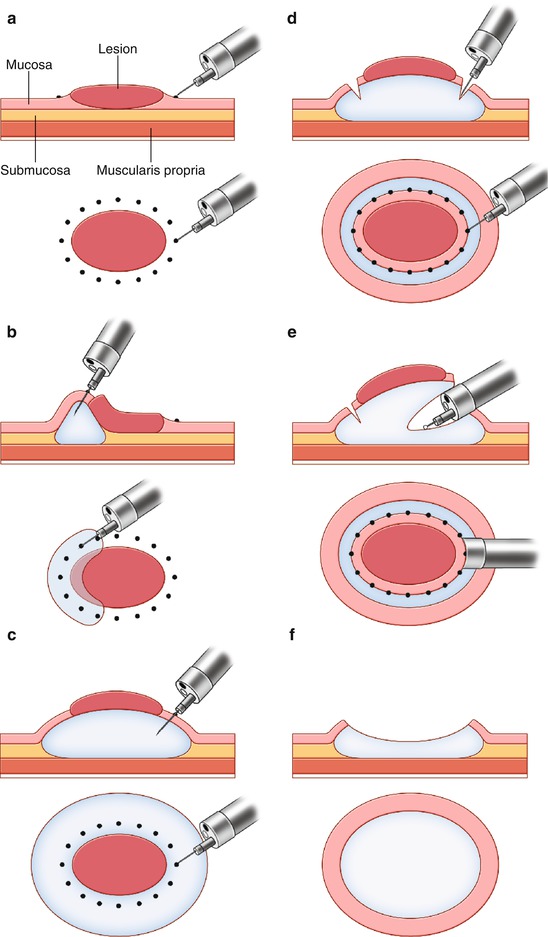
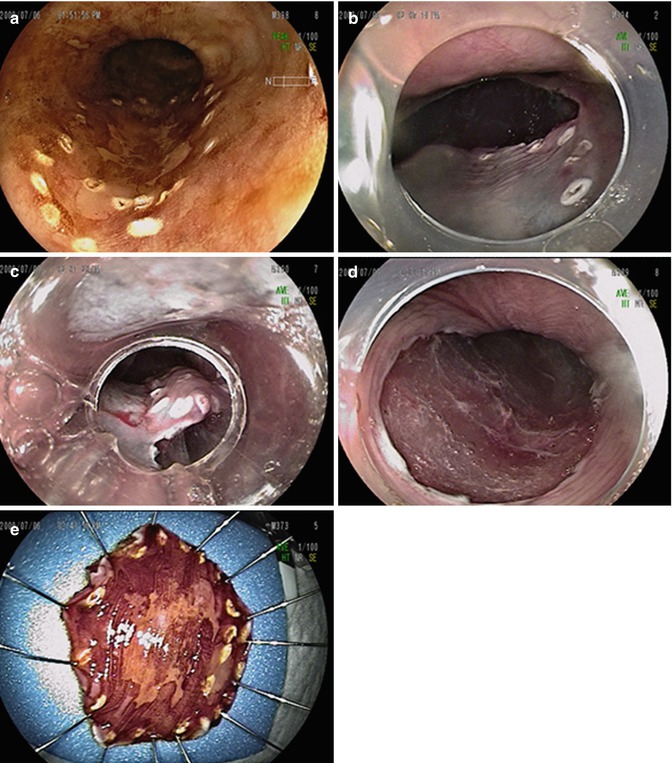

Fig. 2.3
Schematic representation of endoscopic submucosal dissection. (a) Mucosal markings for the incision line. (b) Submucosal injections of a solution. (c) Complete elevation of the lesion by injecting a solution into the submucosal space. (d) Mucosal incision around the mucosal markings. (e) Submucosal dissection with a needle knife through the cap attached on the tip of endoscope. (f) En bloc resection of the tumor. M mucosa, SM submucosa, MP muscularis propria

Fig. 2.4




Endoscopic submucosal dissection of early esophageal squamous cell carcinoma. (a) Chromoendoscopy shows the presence of an irregular unstained area in the middle esophagus. The markings are performed using an electrocautery. (b) After the submucosal injection of sodium hyaluronate, submucosal dissection can be performed. (c) Submucosal dissection is performed using the needle knife. (d) The tumor is resected en bloc. A thin layer of sm3 was observed over the muscle layer. (e) The resected specimen was spread out and pinned on a flat cork
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree