Fig. 7.1
Intragastric balloon
A meta-analysis by Imaz et al. evaluating 15 studies including 3,698 patients with intragastric balloon showed an average weight loss of 14.7 kg, 32.1 % reduction in the excess weight, and decrease of 5.7 kg/m2 in the BMI after 6 months (Imaz et al. 2008). In another review, including 22 studies with a total of 4,371 patients implanted with intragastric balloon, an average weight loss of 17.6 kg was demonstrated, with extremes of 4.9 and 28.5 kg. A greater absolute weight loss was observed in patients with higher BMI (Dumonceau 2008).
A prospective study evaluating the effect of the balloon on weight, insulin resistance, and hepatic steatosis in obese patients showed that 76 % of patients achieved a reduction of 3.5 kg/m2 or more in BMI. The mean weight loss compared to baseline was 16.4 kg (+8.2), with a mean reduction in BMI of 6.4 kg/m2 (±3.2). The absolute percentage of participants with blood glucose levels higher than 100 mg/dl decreased from 50 to 12 %, and the percentage of patients with hypertriglyceridemia greater than 150 mg/dl decreased from 58 to 19 %. Patients presenting changes in ALT also decreased from 38 to 7 % (Forlano et al. 2010).
Regarding follow-up, two studies, one randomized and another uncontrolled, totaling 143 patients, showed that 1 year after gastric balloon removal, these patients had a mean absolute regain of the lost weight of 41 and 28 %, respectively (Herve et al. 2005; Mathus-Vliegen and Tytgat 2005). Another study following 88 patients for a mean period of 22 months after removal observed that 50 % of patients regained some weight, 39 % maintained weight, and only 11 % continued to lose weight after withdrawal (Forlano et al. 2010).
In a European multicentric study comparing findings at baseline, at 6 months after implant (when the balloon was removed) and 3 years after balloon withdrawal, the mean BMI fell from 28.6 ± 0.4 to 25.4 ± 2.6 kg/m2 at 6 months and to 27.0 ± 3.1 kg/m2 at 3 years after removal. The mean percentage of excess weight loss was 55.6 % at 6 months and 29.1 % at 3 years. Patients with hypertension decreased from 29 % at baseline to 16 % at 3 years, diabetes decreased from 15 to 10 %, and dyslipidemia decreased from 20 to 18 %, showing that despite the weight regain, the balloon maintains a benefit in the reduction of the comorbidities (Genco 2013).
The intragastric balloon also has some effect on gut hormones. A prospective analysis including 22 patients with intragastric balloon, evaluating weight, glycated hemoglobin, and the effect on some gut hormones, has been reported. The mean weight loss was 18.4 kg, and it was associated with a significant decrease in glycated hemoglobin. The levels of ghrelin significantly increased after 3 months. Subsequently, the levels of ghrelin decreased but were still above baseline. Leptin significantly decreased, and the levels of adiponectin did not differ significantly (Buzga et al. 2014).
It is important to consider that not all patients have a satisfactory weight loss. Around 20–40 % fail to obtain a significant weight loss (usually defined as >10 % of the initial weight or more than 25 % excess body weight). These may be related to early withdrawal in patients with gastrointestinal or psychological intolerance to the balloon, early disappearance of the effects on hunger and early satiety, or high-calorie food intake (Dumonceau 2008).
The intragastric balloon can be a complement in the treatment of obese patients, facilitating changes in lifestyle, working as an adjunct to drug therapy, and reducing the metabolic complications. Although the balloon does not lead to a sustained long term weight reduction in obese patients, it can facilitate the control of some comorbidities such as diabetes. It can also improve the quality of life in patients with overweight and obesity, who do not want to undergo bariatric surgery.
7.3 Endoscopic Duodenojejunal Bypass Liner (DJBL): EndoBarrier™
A meta-analysis involving 136 papers with 22,094 patients who underwent different techniques of bariatric surgery showed that improvement of type 2 diabetes occurred in 86 % of patients. Analysis of the specific surgical technique reported complete remission of diabetes in 48 % of patients undergoing gastric band placement, 84 % after RYG Bypass, and above 95 % after Bileopancreatic Diversion (BPD) (Buchwald et al. 2004).
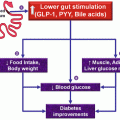
To clarify the reason for the resolution of type 2 diabetes, Rubino et al. (2006) conducted a study comparing diabetic rats who underwent gastric bypass with and without duodenal exclusion. A significant improvement of glycemic control was observed in the group of rats who underwent the duodenal exclusion. Also, when a second operation was performed with duodenal exclusion in the rats submitted to pure gastric bypass, an improvement in glycemic control was observed. Thus, the reestablishment of the food flow through the duodenum in rats with duodenal exclusion was associated with increased levels of glucose.
The analysis of these facts demonstrates that the exclusion of the proximal segment of the small intestine appears to play an important role in improving glucose metabolism. This is the base for developing a device that permits the exclusion of the duodenum, providing a temporary endoscopic duodenojejunal bypass (DJBL) (Levine et al. 2009).
The DJBL is a sterilized, minimally invasive, single-use endoscopic device, which is employed under radioscopic control. It is composed of a nitinol anchoring, with tiny lateral barbs for fixation, and an impermeable plastic conduit made of a fluorine polymer with 62 cm in length, which impedes contact of the chyme with bile–pancreatic secretions prior to the proximal segments of the jejunum (Fig. 7.2). The device is called EndoBarrier™.


Fig. 7.2
Impermeable plastic conduit and anchor system
Didactically, the device has three components: the implant, the system for deployment, and the removal system.
Below the sequence for implantation and removal of the device is observed (Figs. 7.3, 7.4, 7.5, and 7.6)
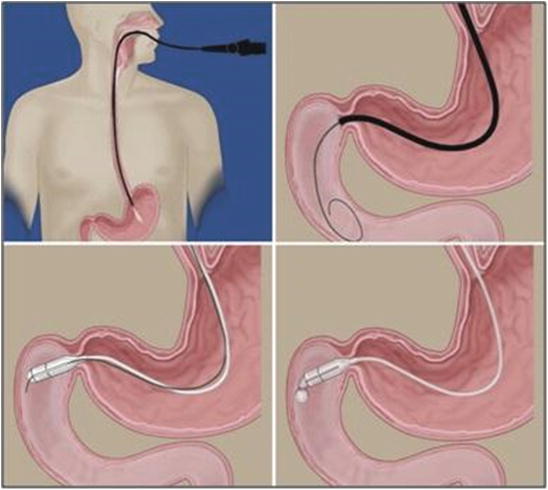
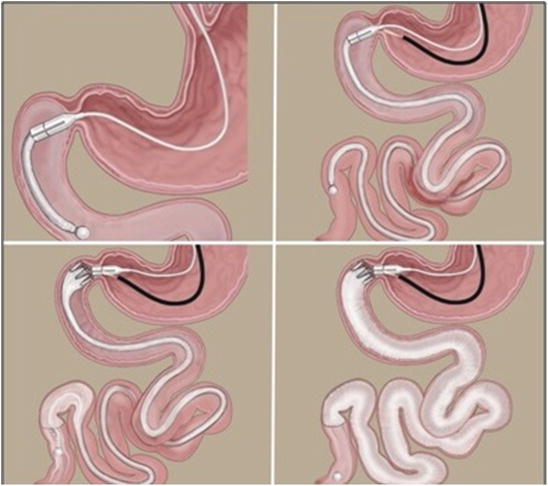
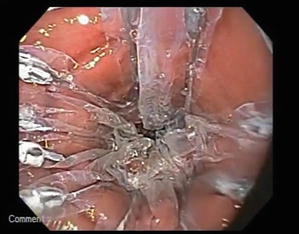
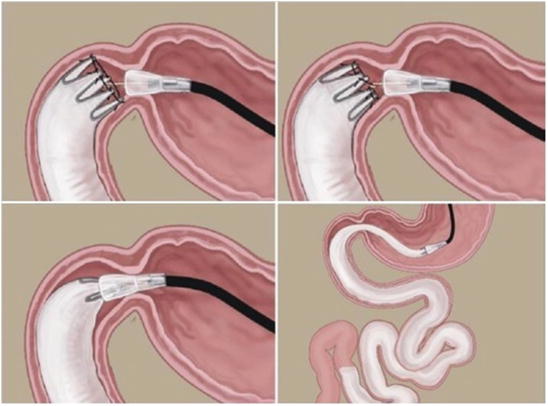

Fig. 7.3
Sequence of DJBL implant: Scope insertion. Guide wire introduction. Introduction of the device over the wire. Starting the release of the sleeve

Fig. 7.4
Sequence of DJBL implant: Progression of the sleeve. Release of the anchor system. Radiologic contrast injection to expand the sleeve and verify patency

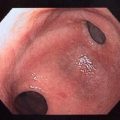
Fig. 7.5
Endoscopic view of recently implanted device

Fig. 7.6
Removal of the device
To evaluate the effectiveness, safety, and tissue reaction, the DJBL was implanted in three groups of laboratory animals (Tarnoff et al. 2008). Four pigs who lived for 90 days, two for 120 days, and three in which no implant was performed, as a control group for the same period. In these series there was one migration and one partial rotation of the device. The tissue reaction caused by the device was defined as mild. There was better control of weight gain in the device group compared to the control, suggesting the effectiveness of the method.
From these studies, started the clinical trials based on the perspective that placing the “DJBL” in duodenal position could mimic some of the features of RYGB surgery, such as the exclusion of the proximal intestine to the flow of food; arrival of nutrients directly to the jejunum; segregation of the digestive secretions; and arrival of partially digested nutrients to the distal gut.
Possible mechanisms of action include malabsorption of calories, alteration of gastrointestinal motility, and modulation of gastrointestinal neurohormonal signaling.
Rodriguez-Grunert et al. (2008) conducted the first human implant and first results publication of 3 months follow-up. This study involved 12 patients (seven women and five men) aged between 28 and 54 years old (M = 41 years old) and BMI between 35 and 51 kg/m2 (M = 42.8 kg/m2), obtaining as result good tolerance and no serious adverse events. The average implantation time was 26.6 min. The patients reported mild abdominal pain and nausea, more concentrated in the first 2 weeks of the implant. After this period the adverse events were related to dietary transgression. All patients lost weight, with an average loss of 23.57 % of initial weight (ranging from 12.5 to 41.5 %), and obtained significant percentage of excess weight loss. The DJBL was removed in two patients due to improper positioning.
A similar result was observed by Gersin et al. (2007), after the publication of the first procedure in the United States. The implant in a female patient, with 36 years and BMI of 45.2, was well tolerated and without complications. The total procedure time was 25 min. The device was removed endoscopically after 3 months, resulting in the total weight loss of 9.09 kg.
Based on these results, the DJBL was approved by the FDA for prospective, blinded, and randomized studies to evaluate their safety and efficacy.
Tarnoff in 2009 published the first randomized, controlled trial for weight loss, involving the implant of the device versus a control group with low calorie diet. The weight loss was higher in the DJBL group, with 22 % of excess weight loss versus 5 % in the control group (p < 0.001), demonstrating effectiveness in achieving short term results. However, only 80 % of the patients completed the 12 weeks implant time. The early removal of the device was performed in five patients: three with hemorrhage, one with migration, and one presenting sleeve obstruction.
In a European multicenter study, the successful implant of the DJBL occurred in 26/30 (86.7 %) patients, and before the end of the protocol the device was removed in 4/26 (15.4 %). The removals were due to migration, displacement of the fixing barbs, sleeve obstruction, and persistent epigastric pain. The average implant time of the device was 35 min (Schouten et al. 2010).
Rodriguez-Grunert et al. (2008) in a further study observed an unplanned effect of the device: the control of type 2 diabetes (T2DM) in patients who are not insulin dependent. This event sparked the interest in the use of this device in a specific protocol for type 2 diabetic patients.
The protocol including only obese diabetic patients was performed at Clinics Hospital of the University of São Paulo, Brazil. Moura et al. 2011b evaluated 22 obese and diabetic patients implanted with the DJBL for a period of 24 weeks. At the end of the study, an average weight loss of 14 kg, BMI decrease of 5.4 kg/m2, and excess weight loss of 22.2 % were obtained. In relation to T2DM, a reduction in fasting blood glucose of 171.8 mg/dl at baseline to 141.5 mg/dl at the end was documented. The glycated hemoglobin (HbA1c) also showed a significant reduction of 8.8–7.3 % (p < 0.001). In this series there were four early explants. Two patients due to causes not related to the device, one with bleeding and the other for persistent abdominal pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree