Mucosal cancer in the gastrointestinal tract generally has low risk of lymph node metastasis. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are techniques of local excision of neoplasia confined to the mucosal layer. Specimens from EMR/ESD contribute to several diagnoses, and histologic results affect treatment decisions. A combined laparoscopic and endoscopic approach to neoplasia with a nonexposure technique allows full-thickness resection of the stomach wall without exposing the gastric lumen to the peritoneal cavity, preventing cancer cell dissemination to the peritoneal cavity. This article reviews EMR/ESD and describes a new full-thickness resection method using the nonexposure technique (CLEAN-NET).
- •
Intramucosal cancer and SM1 cancer (slight sub mucosal invasion) with a low risk of vascular or lymphatic spread with intestinal-type histology are suitable for endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)
- •
Chromoendoscopy (indigo carmine spraying) is useful in the stomach to define the lateral margins of tumors during EMR/ESD
- •
Mucosal lifting by submucosal injection makes EMR/ESD feasible
- •
Deep ulcer scar in the lesion makes it difficult to resect by EMR/ESD
- •
Highly scarred lesions can be totally resected by full-layer resection of the gastric wall
- •
Full-layer resection of gastric wall with non-exposure technique (CLEAN-NET) potentially avoids tumor dissemination
- •
CLEAN-NET combined with regional lymph node dissection may suggest further application of this procedure to submucosal cancer
Surgical resection of gastrointestinal cancers is often associated with significant morbidity and mortality. Therefore, methods for endoscopic cancer resection have been sought. With adequate staging techniques, endoscopic resection of early lesions looks promising. In surgically resected specimens, intramucosal cancer has an extremely small risk of lymph node metastasis. Therefore, endoscopic resection of mucosal cancer is potentially curable without lymph node dissection. EMR/ESD is a technique of local excision of neoplastic lesions confined to the mucosal layer. However, EMR is a snare-based resection method similar to endoscopic polypectomy. The resected margin is often difficult to control accurately. In contrast, ESD is a knife-based dissection procedure in which the resected margin is first identified and cut. The major advantage of ESD is that the lateral margin is sufficiently preserved.
This article discusses the efficacy and limitations of EMR/ESD .
Basic principles of EMR/ESD
The mucosa and muscularis propria are attached to each other by the loose connective tissue of the submucosa. Submucosal injection creates space between them, which allows resection of the mucosa alone, leaving the muscularis propria layer intact. Saline injection into the submucosal layer is the easiest and most cost-effective technique for separating the layers. Lifting of the mucosal surface can be achieved after correct submucosal injection in any part of the gastrointestinal tract. After a sufficient volume of saline has been injected, the mucosa, including the target lesion, can be safely captured and resected by electrocautery or can be dissected by cutting submucosal tissue with an electrocautery knife ( Fig. 1 ).
Brief history of the development of EMR/ESD
Choosing the Appropriate Lesions
Intramucosal cancer with no lymph node metastasis is a definite indication for EMR/ESD. Only mucosal lesions with a low risk of vascular or lymphatic spread are appropriate for EMR/ESD. Accumulated data from surgical lymph node dissections revealed that early stage gastric cancer generally has a low risk of lymph node metastasis. Gotoda and colleagues evaluated the risk of nodal metastasis and advocated ESD for selected gastric lesions. Based on these data, properly selected early stage gastric cancers have no risk of lymph node metastasis (95% confidence). Experts in ESD now consider cases of scarring to be the last unsolved issue in ESD.
Endoscopic ultrasonography is often used to assess lesion depth in conjunction with conventional endoscopy. Magnifying endoscopy is useful to evaluate cancer histopathologic types, but is not useful to assess invasion depth. Biopsy before resection should be a routine procedure because poorly differentiated tumors are more likely to invade deeper layers and often metastasize to lymph nodes, and therefore should be excluded from indications for EMR/ESD. In general, well or moderately differentiated mucosal lesions are considered acceptable targets for EMR/ESD.
Precise evaluation of surgically resected specimens revealed that intestinal-type cancer with slight submucosal invasion (SM1) also has the least risk of lymph node metastasis with no vessel invasion.
Even a flat lesion that contains a poorly differentiated histologic appearance may have a higher risk of invasion beyond the mucosa, and therefore should be resected surgically. Flat lesions with ulcers or scars may have a risk of deeper invasion beyond the mucosa.
Evaluation of Lateral Margins of the Tumor During EMR/ESD
Chromoendoscopy with indigo carmine is useful for defining the lesion before EMR/ESD. The edges of tumor extension may be generally ill defined, particularly with flat lesions. In the stomach, indigo carmine can be used to increase contrast at the interface between normal mucosa and neoplastic lesions. More recently, narrow-band imaging (NBI) magnification endoscopy has been used to distinguish tumor borders from background mucosa by evaluation of surface pattern or vascular pattern. Acetic acid is useful to enhance surface patterns. Once fully visualized, it is useful to mark the edges of the lesions before removal and this can be accomplished with cautery from a snare tip a few millimeters outside the border of the lesion. The presence of cautery markings may be helpful in confirming en bloc resection of a lesion and in reorienting the tissue once it has been removed in multiple fragments.
EMR Techniques
EMR using a cap-fitted endoscope
EMR cap (EMR-C) technique is fast, easy to perform, and can be applied to small lesions (<1 cm). Cap refers to an attachment on the distal tip of the forward-viewing endoscope that is made from a transparent plastic material. The EMR-C technique involves several steps.
Step 1: Preparation
In preparation for the EMR-C procedure, a cap is attached to the tip of the forward-viewing endoscope and is fixed tightly with adhesive tape. For the initial session of EMR in the stomach, an obliquely cut, large-capacity cap with a rim (Olympus MAJ297) is most commonly used by fixing it to the tip of the standard-size endoscope to obtain a larger sample. For trimming a residual lesion, a straight-cut, medium-sized cap with a rim (Olympus MH595) is appropriate. All of the items needed for the EMR-C procedure are commercially available in an EMR kit (Olympus).
Step 2: Markings
The mucosal surface that surrounds the margin of the lesion is carefully marked with cautery using the tip of the snare wire or tip of dual knife (Olympus). Markings are positioned 2 mm from the lesion margin. The color enhancement produced by chromoendoscopy disappears within a couple of minutes, and marking by electrocoagulation is therefore essential, especially for a flat lesion.
Step 3: Injection
A diluted epinephrine-saline solution (0.5 mL 0.1% epinephrine solution in 100 mL normal saline) is injected into the submucosa with an injection needle (23-gauge, tip length 4 mm). Puncturing the target mucosa at a sharp angle is important to avoid transmural penetration of the needle tip. The total volume of injected saline depends on the size of the lesion, but it is necessary to inject enough saline to lift up the entire lesion. Usually, more than 20 mL is injected. When saline is accurately injected into the submucosal layer, lifting of the mucosa or its bulging is observed.
Step 4: Prelooping of the snare wire
A specially designed small-diameter snare (with an outer diameter of 1.8 mm; Olympus SD-221L-25) is essential for the prelooping process. The snare wire is fixed along the rim of the EMR-C cap. To create prelooping conditions, moderate suction is first applied to the normal mucosa to seal the outlet of the cap, and the snare wire that passes through the endoscope instrument channel is then opened. The opened snare wire is fixed along the rim of the cap, and the snare’s outer sheath sticks up to the rim of the cap.
Step 5: Suction of the target mucosa
With the prelooping position maintained, the lesion is fully captured inside the cap and strangulated by simple closure of the prelooped snare wire. At this stage, the strangulated mucosa looks like a snared polypoid lesion.
Step 6: Resection
The pseudopolyp of the strangulated mucosa is cut using blended current electrocautery. The resected specimen can be removed by keeping it inside the cap, without using grasping forceps. The smooth surface of the muscularis propria layer is exposed at the bottom of the EMR-induced ulcer.
Step 7: Additional resection
If additional resection is necessary to remove the residual lesion completely, all the stages, including saline injection, should be repeated step by step. The injected saline usually infiltrates and disappears within a few minutes at the initial injection site, so it no longer acts as a cushion between the mucosa and muscle layer. Repeated saline injection is therefore necessary to reduce the risk of perforation.
EMR using a ligating device (EMR-L) is also a popular procedure. In this procedure, a ligating device for varices is used to create a pseudopolyp. This procedure is technically easy but the specimen is small and limited. One researcher reported a comparative study of EMR-C and EMR-L. They used a small cap for EMR-C. We routinely use a large cap and, therefore, the resected specimen size is larger than with EMR-L.
ESD
EMR was originally based on snare polypectomy. The size of the specimen resected by EMR is limited to a maximum of around 2 to 3 cm. ESD is a novel technique for endoscopic resection that enables 1-piece resection, even for a superficial and widely spreading tumor. In ESD, first described by Ono and colleagues, a knife activated by electrocautery is used both to cut margins and for submucosal dissection beneath isolated mucosa. Devices designed for ESD include the insulation-tip knife, hook knife, dual knife, and flush knife. Each knife has specific features for different applications. The authors prefer to use the flush knife because it has an irrigation function through the outer sheath of the knife. The flush knife irrigation system works both as a submucosal injection needle and for irrigation. It is particularly useful for detecting the bleeding point in cases of bleeding.
Triangle-tip knife procedure
The ESD technique using a triangle-tip knife allows removal of the lesion in a single specimen, even for an extended lesion. The triangle-tip knife is a multipurpose device that can be used for marking, cutting, dissecting, and even hemostasis. Using an edge of the triangle-tip knife, the normal mucosa is marked around the lesion. Injection with high-viscosity solution is mandatory for this procedure because it maintains mucosal lifting for a longer duration. The authors use hyaluronic acid for injection. The energy source is an important factor in performing safe ESD. Swift coagulation (ERBE; Vaio) is considered to be the best electrocautery device for the triangle-tip knife. Marginal cutting about 5 mm outside the markings is the next step. The triangle-tip knife hooks the mucosal edge and pulls the target mucosa away from the surface of the muscle layer, and then cuts it by electrocautery. By repeating the process, circumferential incision around the lesion is completed. Submucosal dissection using the tip of a triangle-tip knife is subsequently performed. A hood mounted on the tip of the endoscope creates a working space beneath the mucosa, and provides countertraction to the tissue in the submucosa. After removal of the mobilized mucosa, complete hemostasis is achieved with coagulation forceps.
Which Procedure Should be Used?
Generally, small lesions can be easily excised with the EMR-C procedure. Gastric lesions smaller than 1 cm can be resected by a single session of EMR-C. ESD is used for larger lesions, to perform en bloc mucosal resections. ESD can be used for any lesions regardless of the size and location of the tumor. The only technical limitation of ESD is for gastric lesions with severe scars, which is easily predicted from signs of nonlifting during submucosal saline injection.
Clinical Results and Complications of EMR/ESD in the Stomach
Many reports from Japan support clinical validity of ESD for M, N0, or SM1N0 lesions. Other reports from Korea, Europe, and Taiwan also support the clinical feasibility of ESD with acceptable risks. Potential complications include bleeding, perforation, and stricture. Bleeding can generally be managed with endoscopic hemostasis using electrocautery forceps. Endoscopic clipping is the last choice to control bleeding from large vessels. Perforation is rare and is treated mainly by endoscopic clipping or conservative management, depending on the size and location of the perforation. Most recently, the Over-The-Scope-clip (Ovesco) is another option for treating perforation. The incidence of delayed perforation is 0.45%, and it should generally be treated by emergency surgery.
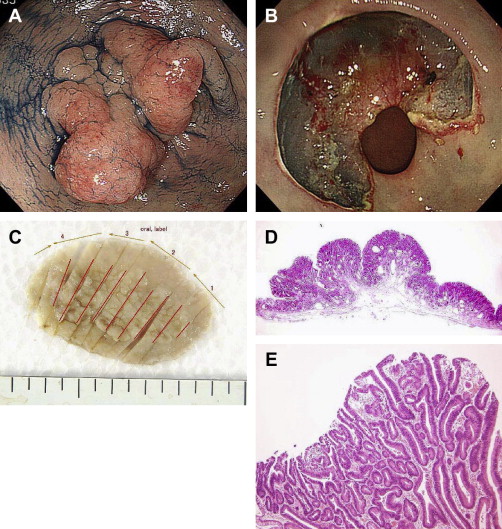
In our institution, a total of 679 lesions in 653 cases received EMR or ESD (from April 2001 to December 2011) ( Box 1 ). In a series of 653 patients, the author’s group used the EMR-C procedure for 181 lesions. No procedure-related death occurred. Perforation occurred in 14 cases (2.0%). In all cases of gastric perforation, the perforated wound was closed using a hemostatic clip. Post-ESD bleeding occurred in 14 cases (2.0%). Bleeding was controlled endoscopically in 13 cases and, in 1 case, it was controlled by angiography. No one required surgery.