Biliary intervention
Preoperative biliary drainage
Palliation of obstructive jaundice
Duodenal intervention
Palliation of duodenal and gastric outlet obstruction
Palliation of pain
Pancreatic duct stent placement
Endoscopic ultrasound-guided celiac plexus/ganglia neurolysis
39.2 Biliary Intervention
Endoscopic biliary intervention in pancreatic cancer includes palliation of biliary obstructions for locally advanced or metastatic disease and preoperative biliary drainage for potentially resectable disease.
39.2.1 Palliation of Biliary Obstruction
Malignant biliary obstruction by pancreatic cancer can present with jaundice and requires palliative drainage if it is unresectable. The mechanisms of biliary obstruction by pancreatic tumor are extrinsic compression, direct tumor infiltration, adjacent inflammation, desmoplastic reaction, or a combination of these factors [6]. Biliary obstruction can develop in as many as 80% during the course of pancreatic cancer if not intervened [7, 8]. Restoration of biliary flow, together with relief of jaundice and pruritus, is the primary goal in the palliation of malignant biliary obstruction, and it also prevents biliary obstruction-related complications such as cholangitis, coagulopathy, malabsorption, and hepatocellular dysfunction [9, 10]. Drainage can be approached in three ways, including surgical bypass (e.g., hepaticojejunostomy or choledochojejunostomy), percutaneous transhepatic biliary drainage, and endoscopic stenting.
A meta-analysis of endoscopic and surgical bypass outcomes in malignant distal biliary obstruction demonstrated the same technical and therapeutic success for endoscopic stenting as for surgical drainage procedures, with similar quality of life and overall survival, but with reduced risk of complications, albeit with an increased risk of recurrent biliary obstruction for endoscopic stenting [11–13]. More treatment sessions are needed after endoscopic stenting than after surgical bypass, but endoscopic stenting still continues to be the most cost-effective approach [14]. Percutaneous transhepatic biliary drainage is associated with considerable morbidity, patient discomfort, and the need for repeated intervention [12].
Endoscopic biliary stenting via endoscopic retrograde cholangiopancreatography (ERCP) is presently the standard of care for the palliation of distal malignant biliary obstruction caused by pancreatic cancer (Fig. 39.1) [11, 12, 15]. It provides effective palliation and may offer lower morbidity and mortality, shorter hospital stay, and diminished overall cost when compared with surgical or radiological approaches [15]. Percutaneous transhepatic biliary drainage is most often used when endoscopic biliary stenting has failed. Endoscopic ultrasound (EUS)-guided biliary drainage can be an effective alternative for percutaneous transhepatic biliary drainage after failed ERCP [16]. Surgical bypass is usually reserved for unsuccessful or unfeasible endoscopic/percutaneous drainage [11].
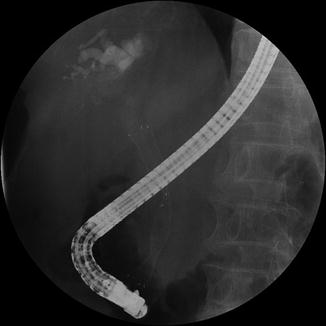

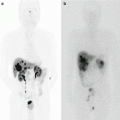
Fig. 39.1
Fluoroscopic image of endoscopically inserted biliary metal stent for a patient with pancreatic head cancer
Various types of stent can be used for endoscopic biliary stenting via ERCP. The biliary stents used for endoscopic palliation include plastic stents and self-expandable metal stents (SEMSs). The SEMSs are classified into uncovered SEMSs and covered SEMSs; the latter are further subclassified into partially covered SEMSs and fully covered SEMSs.
A group of studies comparing between the use of plastic and metal stents in distal malignant biliary obstruction concluded that the patency periods of metal stents are approximately twice those of plastic stents, with a time to first obstruction of 6–10 months vs 3–5 months, respectively [12, 15, 17–19]. SEMS placement is also associated with a lower therapeutic failure, less need for reintervention, lower cholangitis incidence, and decreased hospital readmission but shows no difference in patient survival [20, 21]. Initial insertion of a SEMS (preferably 10 mm of diameter) is preferable as it is more cost-effective if patient life expectancy is longer than 4 months [20]. Initial insertion of a plastic stent (preferably 10 F) is recommended if patient life expectancy is shorter than 4 months or the diagnosis of malignancy is not established.
39.2.2 Preoperative Biliary Drainage
Preoperative biliary drainage has been used for patients with obstructive jaundice by pancreatic head cancer if it is resectable. The theoretical basis of this practice is that surgery in patients with jaundice may increase the risk of postoperative complications [22]. However, as a means of reducing postoperative morbidity and mortality, preoperative biliary drainage has shown conflicting efficacy [22–25]. A recent well-designed randomized trial tested 202 patients with pancreatic head cancer, who were assigned to one of two treatments: (1) surgery alone within 1 week after diagnosis or (2) preoperative biliary drainage with a plastic stent for 4–6 weeks, followed by surgery [26]. The primary outcome was the occurrence of serious complications within 120 days after randomization; these occurred in 39% of patients who underwent early surgery alone and in 74% of patients who underwent preoperative biliary drainage followed by surgery. The rate of occlusion or need for exchange (30%) within a short period in this study was far in excess of routine practice, even then risk of complications associated with routine preoperative biliary decompression in patients with malignant obstructive jaundice and potentially curable cancer of the pancreatic head appeared to outweigh the theoretical benefits of jaundice resolution [27]. Selected patients with suppurative cholangitis or deep obstructive jaundice, or those who awaiting preoperative adjuvant chemotherapy/radiotherapy, may benefit from short-term preoperative biliary drainage. SEMSs are preferred over plastic stents when preoperative biliary drainage is indicated because of their longer patency and better outcome [27, 28].
39.3 Duodenal Intervention
Pancreatic head cancer can invade the duodenum, leading to duodenal and gastric outlet obstruction. This obstruction usually causes nausea and vomiting that becomes intractable, and oral intake eventually becomes markedly reduced or impossible in these patients [8]. Duodenal and gastric outlet obstruction occurs during the course of disease in 10–25% of patients with unresectable pancreatic cancer [8, 29]. Symptoms related to duodenal and gastric outlet obstruction also occur in 10–25% of patients who undergo surgical biliary bypass without gastrojejunostomy during the initial procedure [8]. As a consequence, many surgeons have routinely added gastrojejunostomy at the time of palliative surgical biliary bypass or during exploration of unresectable disease. However, routine gastrojejunostomy is largely being supplanted by an endoscopic placement of duodenal stent.
Duodenal stents used for endoscopic palliation are mostly SEMSs (covered or uncovered) that are 18–22 mm in diameter when fully expanded. Endoscopic insertion of duodenal stents has demonstrated adequate safety and high technical success (approximately 95%), and substantially improves the time to oral intake, which can be as rapid as within 24 h of the procedure [8, 30]. Endoscopic duodenal stenting also resulted in an earlier discharge from hospital and possibly improved survival, when compared with surgical gastrojejunostomy [29].
39.3.1 Palliation of Simultaneous Biliary and Duodenal Obstruction
Surgical diversion of the bile duct and stomach as a one-stage operation has traditionally been performed in cases with dual obstructions of the duodenum and bile duct. Endoscopic palliation of biliary and duodenal obstruction can also be achieved, although this can sometimes be technically difficult [31]. Currently, three types of procedures are recommended in this situation: (1) endoscopic duodenal stenting and EUS-guided biliary drainage (Fig. 39.2), (2) combined endoscopic stenting with duodenal and biliary stents, and (3) endoscopic duodenal stenting and percutaneous biliary drainage. The choice of technique depends on the level of duodenal obstruction, as well as on the local expertise, facilities, and clinical experience.
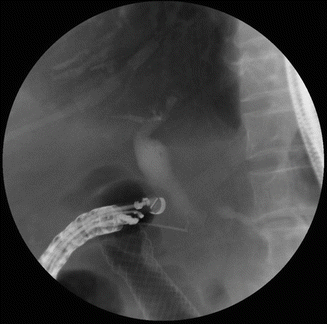

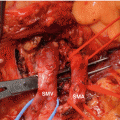
Fig. 39.2
Endoscopic ultrasound-guided cholangiogram was performed after the placement of duodenal stent in a pancreatic cancer patient with duodenal and biliary obstruction. After obtaining cholangiogram, endoscopic choledochoduodenostomy was performed
The location of the duodenal obstruction in relation to the major duodenal papilla may be the major determinant of the success of endoscopic palliation, since a duodenal obstruction can limit access to the biliary orifice [31]. Three duodenal stenosis types are recognized in relation to the major duodenal papilla: (1) at the level of the duodenum proximal to and without involvement of the papilla, (2) affecting the second part of the duodenum with involvement of the major papilla, and (3) involving the third part of the duodenum, distal to and without involvement of the major papilla. Endoscopic dual stenting using a stent-in-stent method can be conducted using a dedicated duodenal stent with a central portion designed to facilitate passage of a biliary stent through the mesh of the duodenal stent (Fig. 39.3) [32]. Nevertheless, the overall survival from the time of combined biliary and duodenal stent placement is relatively short due to disease progression [31, 32].
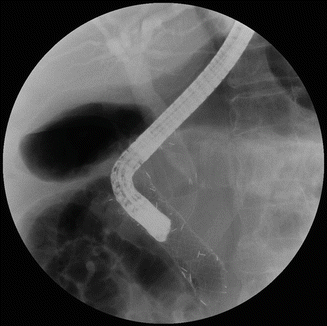

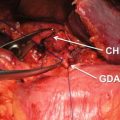
Fig. 39.3
In a pancreatic cancer with duodenal and biliary obstruction, combined endoscopic stenting with duodenal and biliary stents in a stent-in-stent method was performed
39.4 Palliation of Pain
The pain associated with pancreatic cancer can arise due to multiple factors, including perineural encasement by the tumor, invasion of peripancreatic tissues/organs, and obstruction of the main pancreatic duct [33]. Pain due to neoplastic infiltration of the nerve endings in pancreatic and peripancreatic tissues is characterized by chronic, continuous pain of a dull nature, unrelated to meals, located in the upper abdominal quadrants and often radiates to the back [33]. This type of pain is present in the vast majority of advanced pancreatic cancer patients. Pain of obstructive quality may occur in 15% of patients with inoperable advanced pancreatic cancer. This type of pain is characterized by postprandial occurrence, located at the epigastrium and left hypochondrium and radiates to the left back, starting a few minutes after the end of the meal and lasting for 1–2 h; it is associated with a dilated pancreatic duct upstream from the malignant stricture [33, 34].
39.4.1 Pancreatic Duct Intervention
Pain of obstructive quality may be relieved by pancreatic stent placement across the obstructing tumor [34]. The technique used for endoscopic pancreatic stent placement does not differ from that used for the biliary drainage [33]. Large-diameter plastic stents (7–10 F) are preferred for palliation. Steatorrhea associated with ductal obstruction can also be treated by pancreatic stent placement instead of pancreatic enzyme replacement.
39.4.2 EUS-Guided Celiac Plexus/Ganglia Neurolysis
Celiac plexus/ganglia neurolysis has been established as an optional method for providing pain relief and reducing opioid use, because the use of opioid agents is commonly associated with dry mouth, constipation, nausea, vomiting, drowsiness, and delirium [35, 36]. The celiac plexus is located caudal to the diaphragm, surrounds the origin of the celiac trunk, and comprises a dense network of ganglia and interconnecting fibers [36]. The celiac ganglia are predominantly oval or almond shaped with irregular margins, and they vary in number [1–5], diameter (0.5–4.5 cm), and location [36, 37]. The celiac plexus transmits pain sensations from most of intra-abdominal viscera, except the left colon, rectum, and pelvic organs [36, 38]. Advanced pancreatic cancer often infiltrates the retroperitoneal nerves of the upper abdomen, so celiac plexus neurolysis should be considered for pain relief. The celiac plexus can be approached in three ways, for surgical, percutaneous (under fluoroscopic or CT guidance), or EUS-guided neurolysis.
The efficacy of celiac plexus neurolysis is difficult to analyze, due to the mostly retrospective and uncontrolled nature of the studies. A meta-analysis concluded that percutaneous celiac plexus neurolysis leads to successful relief of pancreatic cancer pain [39], whereas another meta-analysis found the data insufficient to judge the efficacy, long-term morbidity, or cost-effectiveness [40]. Eisenberg et al. reviewed 24 studies on percutaneous celiac plexus neurolysis and concluded that (1) celiac plexus neurolysis has a long-lasting benefit for 70–90% of patients with pancreatic and other intra-abdominal cancers; (2) adverse effects are common, but they are transient and mild; and (3) severe adverse effects are uncommon [41]. The limitations of the percutaneous-guided neurolysis are the lack of direct visualization of the celiac trunk, with only approximate accuracy of needle placement, and the risk of vascular puncture and neurologic damage with a posterior approach [42]. A surgical approach termed the intraoperative chemical splanchnicectomy with alcohol also provides pain relief in patients with unresectable pancreatic cancer [43]. Laparoscopic, thoracoscopic, and open surgical approaches can be used, although a surgical approach is rarely undertaken solely for this purpose at present [42].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree