Fig. 8.1
EUS of small pancreatic cancer. EUS shows small hypoechoic area in the head of pancreas
Elastography
Elastography is an imaging modality that can assess the hardness of different tissues and their deformation under compression [10]. In general, pancreatic ductal cancer is well known as the hard tumor including rich fibrosis in the tumor. Pancreatic cancer in the EUS elastography shows predominantly blue, suggesting hard tissue (Fig. 8.2). Recently, the usefulness of elastography by means of has been reported for the diagnosis of pancreatic lesions [10, 11]. However, EUS elastography was not objective at early stage because of the use of elasticity distribution alone. Lately, elasticity semi-quantification, using the strain ratio (SR) of tissue elasticity, is used for objective evaluation [10]. Clinical utility of EUS elastography has been shown by meta-analyses to have a high sensitivity of 95–97% but a low specificity of 67–76% for diagnosing pancreatic cancer [12, 13]. Thus, the improvements in specificity like “measurement of subjective elasticity” appear to be mandatory to become diagnostic standard.
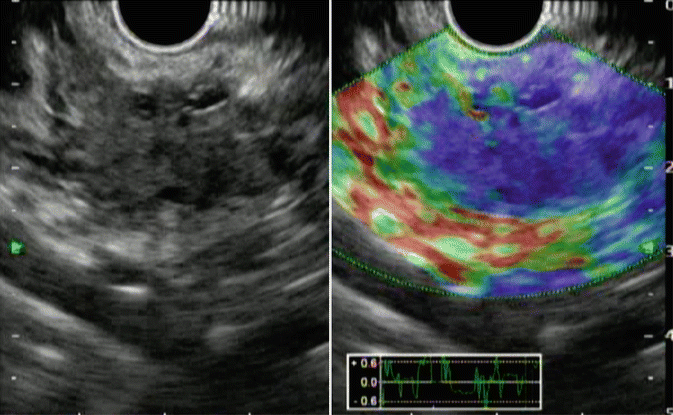

Fig. 8.2
EUS elastography. EUS elastography demonstrates blue area according to pancreatic cancer (left, fundamental image; right, elastography)
Contrast-Enhanced EUS (CE-EUS)
Although fundamental EUS allows the detection of even small pancreatic cancer, it has disadvantage in terms of evaluation of vascularity of the lesions compared with contrast-enhanced CT and MRI. In particular, since pancreatic adenocarcinoma shows hypovascularity, fundamental EUS with color Doppler is not useful unlike for pancreatic neuroendocrine tumor which is hypervascularity tumor. Recently, contrast-enhanced EUS (CE-EUS) using an intravenous contrast agent which characterizes the vascularity of pancreatic masses has been developed [14]. Mostly, pancreatic cancer shows hypovascular pattern in CE-EUS (Fig. 8.3). Furthermore, it aids in not only tumor characteristics but also tumor staging, leading to the guidance of therapeutic procedures. A recent meta-analysis of CE-EUS showed a sensitivity of 94% and a specificity of 89% for diagnosing pancreatic cancer and concluded that it is a promising, reliable modality for the differential diagnosis of pancreatic adenocarcinoma [15]. However, the vascularity pattern of CE-EUS, as well as EUS elastography, is not standardized. Then, one prospective study revealed the usefulness of the quantitative contrast-enhanced harmonic EUS using the use of time-intensity curve (TIC) analysis in an artificial neural network (ANN) classification model [16]. For the ANN, sensitivity was 94.64%, specificity 94.44%, PPV 97.24%, and NPV 89.47% in patients with 112 cases of pancreatic carcinoma and 55 cases of chronic pancreatitis [16].
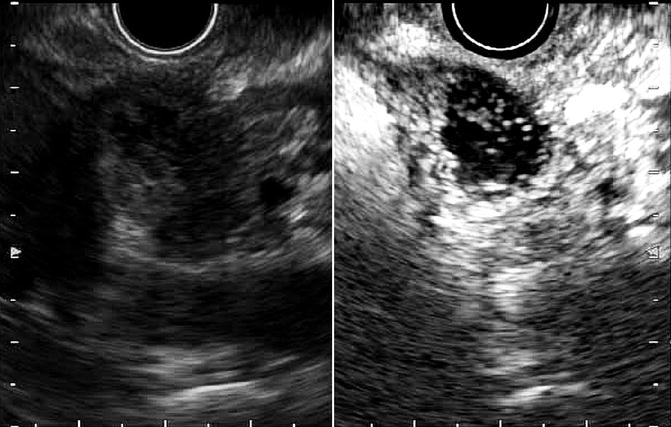

Fig. 8.3
Contrast-enhanced EUS (CE-EUS). CE-EUS demonstrates hypovascular area suggesting pancreatic cancer (left, fundamental image; right, CE-EUS)
Highly diagnostic performance may allow to be replaced with conventional contrast-enhanced CT/MRI in selected patients who have allergy to iodine, renal dysfunction, and metal in the body. Furthermore, in case of difficult EUS-guided fine-needle aspiration (EUS-FNA) like the presence of inevitable large intervening blood vessel in the puncture line, the diagnosis only by CE-EUS seems safe and valuable to avoid unnecessary complication.
8.1.1.2 EUS-FNA
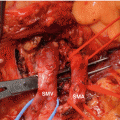
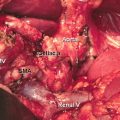
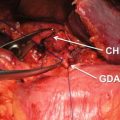
EUS-FNA, which emerged for diagnosis of pancreatic lesion in 1992 [17], has a high diagnostic ability for pancreatic cancer because it allows not only precise images but also sampling for pathological diagnosis (Fig. 8.4). The diagnostic accuracy of EUS-FNA is 85–90% in high-volume centers in the world [18–22]. Recent meta-analyses of EUS-FNA demonstrated its high sensitivity of 86.8–91% and specificity of 94–99.3% for diagnosing pancreatic masses [23–25]. Thus, nowadays, despite the presence of resectability, pathological sampling by EUS-FNA is standard diagnostic strategy when pancreatic masses are detected by imaging modalities like CT and MRI. However, EUS-FNA has several points of weakness. Standard EUS-FNA technique, e.g., selection of needle, sampling technique (funning technique, etc.), and the presence of on-site pathologist (rapid on-site evaluation, ROSE), is not established yet. Furthermore, it is likely that the outcome of EUS-FNA depends on the endosonographers’ skill. Although transabdominal ultrasound (US) also depends on operator’s skill, interestingly sequential comparative study in the same institution showed that EUS-FNA can obtain significantly adequate specimens compared with US-FNA (100% vs 91.3%, p = 0.019), and diagnostic accuracy by EUS-FNA cytology was significantly superior to that of US-FNA (94.6% vs 78.6%, p = 0.0079), though there was no significance on the serious adverse events rate between EUS-FNA and US-FNA (1.3% vs 4.3%) [26]. Theoretically, small pancreatic mass may preclude adequate pathological sampling. In fact, one study in high-volume center revealed that size of mass affected diagnostic yield of EUS-FNA in patients with pancreatic masses (accuracy: <1 cm, 47.4%; 1–2 cm, 78.9%; 2–3 cm, 86.9%; 3–4 cm, 92.6%) [27].
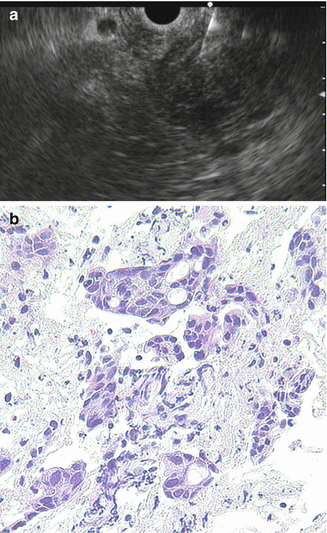

Fig. 8.4
EUS-FNA of pancreatic head cancer. (a) A 22-gage fine needle is advanced into the pancreatic head mass. (b) Histology (H&E staining)
Multiple gene abnormalities influence the progress of pancreatic cancer. Until now, several investigators suggested that sample analyses obtained by EUS-FNA are useful not only for diagnosis of pancreatic cancer [28, 29] but also selection of therapeutic strategy even in advanced pancreatic cancer [30]. Thus, the progress of genetic technology may allow tailor-made medicine in patients with pancreatic cancer.
8.1.2 Endoscopic Retrograde Cholangiopancreatography (ERCP)
First endoscopic retrograde pancreatography (ERP) was reported by Mucune et al. in 1968 [31]. Since then, ERCP has been used for diagnosis and therapy of pancreatic cancer. Theoretically, pancreatic ductal carcinoma seems to be originated from main or branch pancreatic duct. Thus, it shows morphologic change of pancreatic duct like disruption, stricture, and dilation (Fig. 8.5). ERP enables not only possibility of the presence of pancreatic cancer but also obtaining pathological sampling in the pancreatic duct. If cancer has invasion to the bile duct, resulting obstructive jaundice, ERC shows bile duct stricture and shift of bile duct to the pancreatic side. In general, since biliary stent is placed across the biliary stricture following diagnostic ERCP, sampling by brush cytology and transpapillary biopsy is usually performed before stent placement. However, the most worrisome problem with ERCP is the development of procedure-related complications particularly post-ERCP pancreatitis, though it may not be so many in case of head of pancreatic cancer because of few intact pancreatic duct. Thus, with MRCP development, the use of simple ERCP has considerably decreased only as a diagnostic tool unless therapeutic ERCP like biliary stenting is needed.
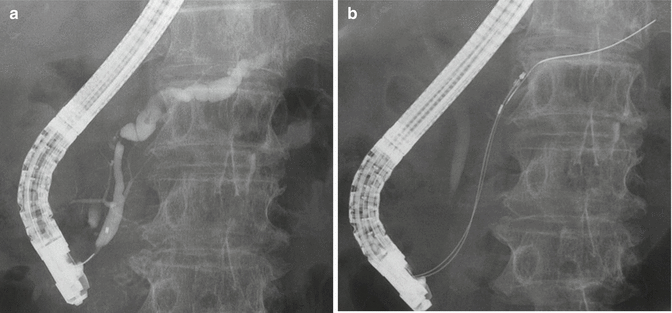
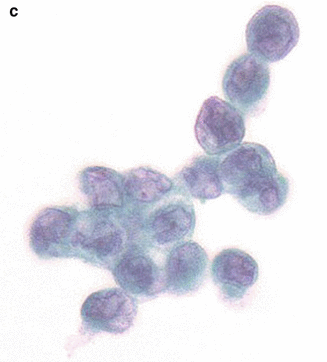


Fig. 8.5
Endoscopic retrograde pancreatography (ERP). (a) ERP showed main pancreatic duct stricture. (b) Brushing cytology was conducted. (c) Cytological specimen showed malignant cells
Recently, one worrisome paper described that cytodiagnosis of pancreatic juice may be useful in the diagnosis of pancreatic carcinoma in situ [32]. However, such kind of invasive diagnostic ERCP should be performed based on the benefit and harm for the patients. Nevertheless, ERCP may have small potential as a diagnostic modality in combination with EUS. Another interesting study showed that the ERCP and EUS combination was associated with a high diagnostic value for detecting pancreatic neoplasms compared with ERCP or EUS alone for pancreatic solid lesions [33].
Intraductal ultrasonography (IDUS) had been performed more than one decade ago for diagnosis of pancreatobiliary strictures. However, catheter mostly cannot pass the stricture and provide additional information compared with conventional EUS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree