This article focuses on endoscopic and robotic surgical techniques for the treatment of thyroid cancer. The most widely applied techniques are minimally invasive video-assisted thyroidectomy, as well as various combinations using a primarily axillary-based approach. Although there are few large studies delineating the benefits of endoscopic thyroid resection when compared with traditional thyroidectomy, in patients who desire the lack of a cervical incision, endoscopic thyroidectomy provides a safe and oncologically effective surgical option when applied by experienced surgeons. Endoscopic surgical treatment of thyroid disease is a developing field and deserves further study before widespread application.
Key Points
- •
Minimally invasive approaches for the surgical treatment of thyroid cancer are under investigation as a result of improvements in technology and the desire to avoid visible neck incisions.
- •
Multiple minimally invasive approaches to thyroid surgery have been used, including endoscopic thyroidectomy and robotic thyroidectomy. There is no clearly superior approach.
- •
Endoscopic and robotic thyroidectomy are still in the investigational phase, and further study is required to determine the appropriate context for the application of these techniques in the clinical setting.
Introduction
Thyroid cancer is the most common cancer of the endocrine system, and the fifth most common cancer affecting women in the United States, with approximately 56,000 expected new cases in 2012. From 1999 to 2008, the incidence in thyroid cancer increased significantly, with the greatest increase noted in the diagnosis of localized disease, from 5.2 to 9.6 cases per 100,000 people. This increase is largely believed to be related to improvements in diagnostic imaging, given that the largest increase has been observed in lesions less than 2.0 cm. Most thyroid cancer is considered to be well-differentiated thyroid cancer (DTC), which includes papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and Hurthle cell carcinoma, with PTC comprising most of these cases. PTC and FTC have a favorable prognosis with 5-year survival of ∼97% ( http://www.cancer.gov/ , 2012) and share recommendations regarding surgical therapy. Given that the greatest increase in thyroid cancer has been in patients with localized disease, the number of patients seeking surgical consultation for the treatment of thyroid cancer is expected to continue to increase.
Recently, the American Thyroid Association (ATA) published revised evidence-based guidelines for the treatment of thyroid cancer, including surgical therapy. Despite the acceptance of these guidelines by multiple endocrinology and endocrine surgery associations, controversies remain regarding optimal surgical therapy. The 2 main areas of controversy related to surgical therapy include the extent of thyroid resection (thyroid lobectomy vs total thyroidectomy) and the use of prophylactic central neck dissection (CND) in low-risk PTC, and papillary thyroid microcarcinoma (<10 mm, PTMC). It was recently shown in a large study of the American College of Surgeons National Cancer Data Base (more than 50,000 patients) that for PTC greater than 1 cm, total thyroidectomy decreased risk of recurrence and death when compared with those patients who underwent lobectomy; however, there was no difference in patients with PTC less than 1 cm. A large study of the SEER (Surveillance Epidemiology and End Results) database from 1983 to 2002 showed no difference in 10-year overall survival or cancer-specific survival in patients undergoing thyroid lobectomy or total thyroidectomy for DTC, although multivariate analysis suggested that total thyroidectomy was superior. Despite these results, controversy remains, because other studies have shown that tumor size has no effect on the incidence of contralateral disease, and the clinical significance of contralateral disease is unclear. The current ATA guidelines recommend that patients with a preoperative diagnosis of PTC greater than 1 cm undergo total or near-total thyroidectomy 4 ; however, the appropriate surgical treatment of patients with PTMC remains to be defined.
Another area of considerable controversy in surgical management of DTC is whether or not to perform a prophylactic CND. The relevance of CND for patients with clinically or radiographically evident nodes is well established. In addition, the ATA guidelines suggest that routine prophylactic CND may be performed for patients with T3 or T4 tumors, because patients with lesions 4 cm or greater have an increased risk of nodal metastases ; however, for patients with tumors less than 4 cm, the evidence for prophylactic CND is less clear. Many patients with PTC with clinically node-negative disease undergoing prophylactic CND have occult positive nodes, and prophylactic CND reduces postoperative thyroglobulin levels, but in a recently published meta-analysis, CND was not associated with a significant decrease in local recurrence. Prophylactic CND does seem to increase postoperative morbidity, but the information obtained from prophylactic CND may provide important staging information, because lymph node status was an independent predictor of survival in a recent study of the SEER database. Clearly, this is an area in need of further study to effectively define which patients most benefit from CND.
Historical perspective
Thyroid surgery has seen many advances over the years. Thyroid disease has been recognized throughout much of human history, beginning with early writings from China regarding the use of seaweed to treat goiter in 2700 bc . Early thyroid operations, before the advent of modern techniques and asepsis, were performed largely only because of morbidity associated with goiters causing airway obstruction. Dr Samuel Gross wrote in 1866, “every step the surgeon takes will be environed with difficulty; every stroke of his knife will be followed by a torrent of blood, and lucky will it be for him if his victim lives long enough to enable him to finish his horrid butchery.” Because of the early difficulty with thyroidectomy, it is not surprising that some of the most recognized surgeons in history made significant advances in thyroid surgery, including Theodor Billroth, Theodor Kocher, William Steward Halsted, and Charles Mayo. Dr Kocher was awarded the 1909 Nobel Prize for Medicine for his pioneering work in thyroid physiology and surgery. The legacy of innovation and progress in thyroid surgery has continued through the modern era. Currently, this innovation is most notable in the form of minimally invasive techniques for the treatment of thyroid disease and thyroid cancer. In this article, the application of these techniques is discussed, including endoscopic and robotic surgery for thyroid disease.
Early advances in minimally invasive, or less-invasive, techniques of thyroid surgery involved using smaller open incisions, evolving from 8-cm to 10-cm incisions to the use of 3-cm to 5-cm incisions. The introduction and widespread adoption of endoscopic technology then led to the use of this technology in thyroid surgery. Although the open approach is expeditious, and the incision is generally well hidden in a cervical neck crease, possible advantages of endoscopic approaches include improved cosmesis and, given the use of endoscopes with magnification, potentially improved visualization. Currently, several different methods of endoscopic approach to the thyroid gland are practiced, and we review the different techniques and the current literature about these various approaches ( Table 1 ).
| Technique | Year | Port Sites | Camera Position | References |
|---|---|---|---|---|
| Minimally invasive video-assisted thyroidectomy | 1999 |
| Suprasternal | |
| Endoscopic breast approach | 2000 |
| Ipsilateral parasternal | |
| Endoscopic transaxillary approach | 2001 |
| Axilla | |
| Endoscopic anterior chest approach | 2002 |
| Inferior ipsilateral clavicle | |
| Endoscopic axillobilateral breast approach | 2003 |
| Axilla | |
| Robotic anterior chest axillary approach | 2009 |
| Axilla | |
| Robotic bilateral axillary bilateral areolar approach | 2009 |
| Right supra-areolar | |
| Robotic unilateral transaxillary approach | 2010 |
| Axilla | |
| Robotic retroauricular face-lift incision approach | 2011 |
| Postauricular crease |
Endoscopic thyroidectomy
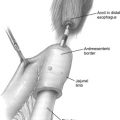
The first endoscopic thyroid surgery was reported by Huscher and colleagues, when they described the use of an anterior cervical approach to perform a right thyroid lobectomy. This lobectomy was accomplished using 3-mm to 5-mm ports. One port was at the jugular notch, one was at the angle of the mandible, and one was midway between the other 2. These investigators used a 30 o endoscope and CO 2 insufflation to develop a dissection plane in the subplatysmal space. Since this initial description, various other techniques for endoscopic removal of the thyroid have been described. The most commonly used procedure in North America is the minimally invasive video-assisted thyroidectomy (MIVAT). This procedure was initially described for the treatment of small thyroid nodules in 1999 by Miccoli and colleagues. Several alternative approaches to endoscopic thyroidectomy have been described as well. The transaxillary approach was initially described in 2001 by Ikeda and colleagues for unilateral thyroid lesions and has been adopted by some groups, both unilaterally and bilaterally, for endoscopic as well as robotic thyroidectomy. In addition, various other extracervical approaches have been described, some as combinations of other techniques. These approaches include an anterior chest approach, a breast approach, and the axillobilateral breast approach (ABBA). Feasibility and limited clinical studies have also been described using a retroauricular approach, a dorsal approach, and a transoral, incisionless approach for both endoscopic and robotic thyroidectomy. Here we discuss some of the technical aspects of the most commonly used cervical techniques (the MIVAT approach), as well as some aspects of the more widely used extracervical techniques including the transaxillary approach.
The MIVAT approach, as originally described for thyroid lobectomy, is accomplished by first making a 15-mm incision 2 cm superior to the sternal notch, followed by careful dissection in the subplatysmal plane. The linea alba is then incised for approximately 3 to 4 cm. A 12-mm trocar is then introduced between the strap muscles and the thyroid lobe, and CO 2 insufflation is applied under direct visualization with a 30 o 5-mm endoscope to 12 mm Hg for approximately 3 minutes to further develop the dissection plane between the thyroid and the strap muscles, opening the thyrotracheal groove. After this time, insufflation is allowed to fully egress. Needlescopic 2-mm forceps and aspirator are then introduced under direct visualization through a small supraclavicular incision and 2-mm scissors or a spatula are introduced through the main incision. The dissection is then carried out laterally to medially, carefully identifying the parathyroid glands and recurrent laryngeal nerve (RLN). Bipolar cautery is used for hemostasis on smaller vessels away from the RLN and clips are used on larger vessels and those near the RLN. Once the thyroid is mobilized from these important structures, the dissection is carried out in standard fashion, removing the gland from the trachea. To complete a lobectomy, the isthmus is then dissected and divided from the contralateral lobe by running absorbable sutures. The specimen is then removed from the main incision, hemostasis is confirmed, and the linea alba and platysma are closed with absorbable sutures. Generally, no drains are left in place. The generally accepted indications for MIVAT are as follows: benign thyroid nodules, the largest diameter of which is less than 35 mm, cytologically malignant nodules less than 20 mm, and an ultrasonographically estimated thyroid volume less than 25 cm 3 . It is also believed that the presence of either severe thyroiditis, or preoperative suspicion of metastasis to either the lateral or central neck lymph nodes, are contraindications to performing MIVAT; however, it has been recently suggested that these contraindications may be unfounded. In a series of nearly 2000 patients (511 thyroid lobectomy, 1435 total thyroidectomy) recently published by Minuto and colleagues, it was found that up to 30% of patients with PTC had thyroiditis on final pathologic examination and only 1 case was converted to open because of thyroiditis. In addition, these investigators found that the mean number of nodes removed in patients with suspected central neck metastases was similar to the number removed in open operations in their hands.
The transaxillary endoscopic approach to thyroidectomy was originally developed to provide a safe operative approach for patients concerned with the cosmesis of a neck incision. The patient is positioned with arm(s) out depending on the affected side or bilaterality of the operation. Generally, a 1.5-cm to 3-cm incision is made in the axilla and the platysma is exposed through the upper portion of the pectoralis major muscle or via tunneling subcutaneously. Conventional 12-mm and 5-mm trocars are placed in the axillary incision, the trocars are then purse-stringed in place, and a low insufflation pressure (3–6 mm Hg) is applied for a few minutes. After adequate insufflation is achieved, 1 to 2 additional ports are placed in the axilla to allow access for dissection instruments. The thyroid is then exposed by dissection of the sternocleidomastoid from the strap muscles (sternohyoid and sternothyroid). The thyroid dissection then progresses as described earlier, with careful identification of the RLN and superior and inferior parathyroid glands. Once the thyroid has been sufficiently mobilized and the key structures identified, the thyroid is then dissected off the trachea, and the isthmus is transected using the harmonic scalpel for completion of a lobectomy. The specimen is then extracted via the main axillary incision, a closed suction drain is left in the subplatysmal space, and the skin is closed in standard fashion. Modifications of this approach that include breast incisions (Refs. ABBA) typically use bilateral superior areolar incisions. In the ABBA approach, the endoscope is typically inserted via one of the areolar incisions and most of the dissection is accomplished via instruments introduced through the axillary incision(s).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree