Outline
Endometrial Hyperplasia: Pathologic Diagnostic Criteria
Management Decisions for Atypical Endometrial Hyperplasia (Endometrial Intraepithelial Neoplasia)
Management of Endometrial Hyperplasia Without Atypia
Prevention of Endometrial Cancer
Benefits and Risks of Estrogen Replacement Therapy
Estrogen Replacement Therapy for Endometrial and Breast Cancer Survivors
Key Points
- 1.
Atypia is the most worrisome feature in endometrial hyperplasia.
- 2.
Complex hyperplasia with atypia has a concomitant risk of malignancy of more than 40%, many of which are high grade.
- 3.
Estrogen therapy is the most effective treatment of postmenopausal vasomotor symptoms.
- 4.
Estrogen is effective in the prevention and treatment of osteoporosis.
Introduction
The endometrium is a very dynamic tissue in reproductive age women. It is continuously changing in response to hormonal, stromal, and vascular influences, with the intended goal of implanting an embryo and supporting the nutritional needs of the developing pregnancy. Whereas estrogen stimulation is associated with the growth of proliferative endometrium ( Fig. 4.1 ), progesterone produced by the corpus luteum after ovulation inhibits proliferation and stimulates formation of secretory endometrium ( Fig. 4.2 ). Without conception and human chorionic gonadotropin (hCG) production, the corpus luteum fails to produce progesterone, and hormonal withdrawal allows endometrial breakdown and menses to occur. Continuous estrogen stimulation of the endometrium bypasses the normal recycling of the endometrium. Menarche and menopause represent transition times of varying lengths in women’s lives when the absence of ovulation predisposes them to unopposed estrogen stimulation because no corpus luteum forms to secrete progesterone. Some women (≈5%) have polycystic ovarian disease, which is a prolonged anovulatory condition prone to estrogen excess. Obesity during menopause also produces a state of excess estrogen production. This is due to the peripheral conversion, in the adipose tissues, of androgens secreted from the adrenal glands and ovaries into an estrogen, estrone, by the enzyme aromatase.
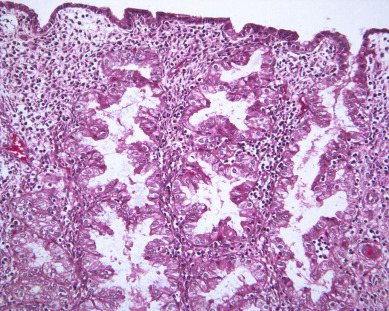
Unopposed estrogen stimulation yields a continuous spectrum of change from proliferative endometrium ( Fig. 4.3 ) through many variations of endometrial hyperplasia until a malignant neoplasm develops. Endometrial cancer is defined by the ability to invade local tissue and metastasize ( Fig. 4.4 ). Endometrial lesions show glandular and stromal variations in continuous change, presenting the challenge of classifying endometrial hyperplasia into distinct, reproducible categories that predict neoplastic risk, as well as response to progesterone therapy. Histopathologic classification of endometrial lesions provides a stratification of risk for progression to cancer by defined endometrial changes.
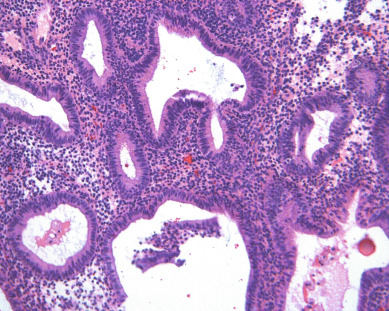
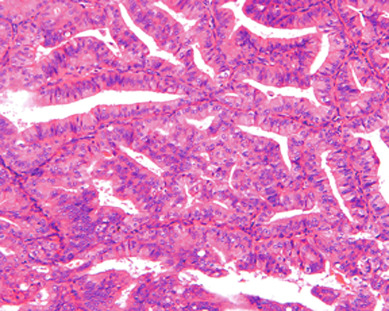
Understanding the pathophysiology of excess unopposed estrogen production helps the clinician better predict which women are at risk for endometrial cancer and provides windows of opportunity for implementation of prevention strategies. Selective estrogen receptor modulators (SERMs) such as tamoxifen and raloxifene and, more recently, aromatase inhibitors are widely used as both treatment and chemoprevention of breast cancer. These drugs have toxicities that the obstetrician/gynecologist will be required to manage. Although tamoxifen is an antiestrogen in breast tissue, it paradoxically has estrogen-like properties in the endometrium and increases the risk of endometrial cancer. It is also associated with thromboembolism and stroke. Although tamoxifen provides protection against bone loss, osteoporosis, and fractures, it does not appear to carry increased risk of myocardial infarction. There does appear to be an association with cataracts. Aromatase inhibitors block the cytochrome p450 CYP-19 enzyme responsible for the peripheral conversion of androgens to estrogens and result in estrogen deficiency. This accelerates menopausal bone loss, which could lead to osteoporosis and fractures; therefore, prevention of these toxicities is important when they are prescribed. Other side effects include hot flashes, sweats, edema, myalgias, and fatigue. Unfortunately, both tamoxifen and aromatase inhibitors accentuate menopausal symptoms.
Preventive health maintenance must balance all risks and benefits while maintaining quality of life and symptom control, and this is the central theme in this chapter. Many women want active control over these very important choices, balancing quality of life and their sexuality with factors such as cancer prevention and cardiovascular risk reduction.
Clinical Presentation
The scientific explanation for the low incidence rates for endometrial hyperplasia is that it is a lesion that is usually unrecognized and asymptomatic until cancer develops. Until endometrial cancer screening becomes routine and cost effective, the true prevalence of the precursor lesions will remain unknown. Women with endometrial hyperplasias are identified by endometrial biopsy performed because of abnormal vaginal bleeding (menorrhagia or postmenopausal bleeding) or because a thickened endometrial stripe is found on transvaginal ultrasonography when ordered for another reason such as the identification of endometrial cells in the Pap test of a woman older than the age of 40 years.
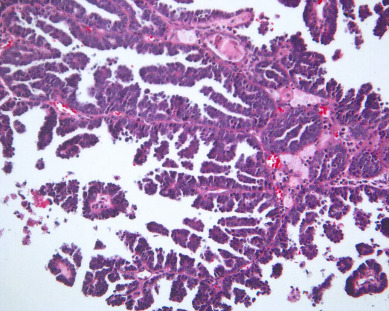
There is evidence of exogenous or endogenous unopposed estrogen stimulation of the endometrium in most women with endometrial hyperplasia. The most common causes of excess estrogen are obesity, polycystic ovarian disease, or a prolonged perimenopause with anovulatory bleeding patterns. Some women have an ovarian neoplasm producing the estrogen stimulation, which is the classic presentation of a granulosa cell tumor. The development of hyperplasia secondary to anovulation at menarche is very uncommon and should be easily reversible, with normalization of cyclic menses with oral contraceptive pills. The age at presentation depends on the source of the excess estrogen. Endometrial hyperplasias and cancers that are associated with estrogen stimulation have a good prognosis. Endometrial cancers in women without evidence of excess estrogen are usually not associated with hyperplasia and can be associated with a tumor suppressor gene abnormality such as p53 associated uterine papillary serous carcinomas ( Figs. 4.5 and 4.6 ). These are aggressive tumors, behaving similar to ovarian cancer with higher mortality rates.
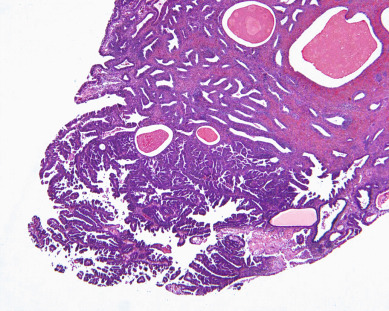
Office endometrial biopsy has replaced the dilation and curettage (D&C) procedure for the diagnosis of endometrial hyperplasia or carcinoma. Historically, there was thought to be potential benefit of curettage as therapy, as well as providing more tissue for accurate histologic diagnosis. The use of “hormonal curettage” in which medical therapy with progesterone is followed by the withdrawal bleed provides the same benefit without the surgical cost or risk. Repeat office endometrial biopsy at 3-month intervals can reassure the patient and the clinician of the therapeutic effect of the hormonal therapy. An argument can be made that hysteroscopy and D&C for abnormal bleeding would potentially be more effective in identifying endometrial cancers, but there is no evidence that D&C reduces a woman’s mortality risk. This outpatient office evaluation and management has become the usual and safest way to manage these patients until there is evidence that a hysterectomy is required. These management decisions are discussed further in later sections of this chapter.
Endometrial Hyperplasia: Pathologic Diagnostic Criteria
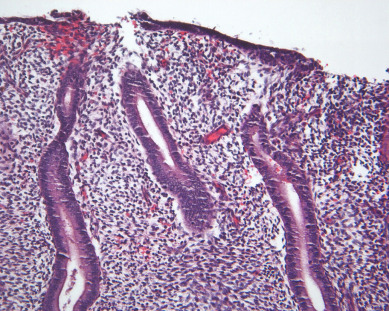
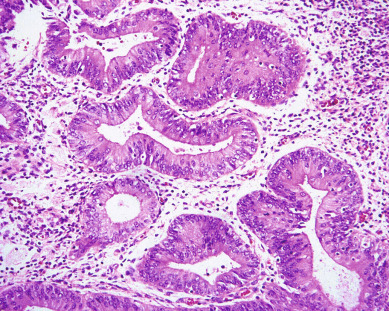
There are currently two systems of nomenclature for endometrial precursors, the 1994 World Health Organization (WHO94) schema and the endometrial intraepithelial neoplasia (EIN) system. Recently, the American College of Obstetrics and Gynecology and the Society of Gynecologic Oncology have published their “Committee Opinion” endorsing the EIN system but the community pathologists are not yet using this system. The International Society of Gynecological Pathologists (ISGYP), International Federation of Gynecology and Obstetrics (FIGO), a schema is commonly used to classify endometrial hyperplasia into four categories based on architectural structure and cytologic features. The architecture is either simple or complex, and the cytologic features are described as with or without atypia. This yields four separate diagnoses ( Table 4.1 ): simple hyperplasia without atypia (see Fig. 4.3 ), complex hyperplasia without atypia, simple hyperplasia with atypia, and complex hyperplasia with atypia (AEH) ( Fig. 4.7 ). The terms adenomatous and cystic-glandular hyperplasia have been discarded, and when the abbreviation AEH is used, it refers to atypical endometrial hyperplasia (AEH), which reflects the two categories of hyperplasia with cytologic atypia (simple hyperplasia or complex hyperplasia with atypia). The presence of atypia appears to be the most important criteria for progression to adenocarcinoma or the coexistence of endometrioid adenocarcinoma. The rates of coexisting endometrioid adenocarcinoma with AEH are reported to be as low as 13% and as high as 43%.
|
The pathologic diagnosis of these lesions is usually made with a small sampling of the endometrium in the office using an endometrial biopsy device. This small tissue sample then has to be categorized by the pathologist. A prospective study of the reproducibility of the diagnosis of AEH (both simple and complex) was undertaken by the Gynecologic Oncology Group (GOG). Women with the diagnosis of AEH who agreed to have a hysterectomy within 12 weeks of diagnosis of AEH were enrolled. The study population included 302 eligible cases with a median age of 57 years; 31% were 50 years of age or younger. The endometrial biopsy specimens were re-reviewed by three gynecologic pathologists independently (study panel diagnosis) in a blinded protocol. They agreed to categorize the endometrium as normal (cycling, menstrual, or atrophic), nonatypical hyperplasia (disordered proliferative, simple or complex hyperplasia), atypical hyperplasia (simple or complex), adenocarcinoma, or inadequate for evaluation. Two of three study pathologists had to agree on one of the above five diagnoses for a study panel diagnosis to be established. Correct diagnosis was determined by consensus agreement on the hysterectomy specimen during a simultaneous panel review with a multiheaded microscope. The results found only 40% of the cases had all three pathologists in agreement. The reproducibility was lowest for the diagnosis of AEH (kappa 0.28), and better for adenocarcinoma (kappa 0.51). Reproducibility was best for D&C (kappa 0.47; confidence interval [CI] 0.41:0.53) compared with small sampling devices (kappa 0.26–0.36). Two of three study panel members agreed with the referring institution diagnosis of AEH in 38% of cases. The study panel diagnosis was less severe in 25% of cases and adenocarcinoma in 29% of cases. The most important outcome for this study was the finding that 43% of the enrolled participants were found to have adenocarcinoma in their uteruses at the time of hysterectomy. The second most important finding was the rate of adenocarcinoma in each study panel majority diagnosis category: normal or nonatypical hyperplasia, 14 of 74 (18.9%); AEH, 45 of 115 (39.1%); and adenocarcinoma, 54 of 84 (64.3%). The uterine examination revealed the presence of some risk factors for metastatic disease present in 43 cases, including myometrial invasion or grade 2 or 3 lesions.
The natural history of simple hyperplasia without atypia is likely to follow a benign course. It is often seen in women near menopause when anovulatory cycles are common. The removal of the estrogenic stimulation or treatment with progestogens favorably influences the outcome. The majority of lesions will regress (60%) without treatment, and 84% will regress with progestin therapy. Only 3% are believed to progress to cancer. In the absence of atypia, the goal of treatment is to prevent progression to cancer in a small population of patients and to control abnormal uterine bleeding. The duration of treatment and best agent for progestin therapy have not yet been identified; however, medroxyprogesterone acetate (MPA) is typically used because there is the most clinical experience with it. Other progestin preparations that have been effectively used in hyperplasia without atypia include levonorgestrel-releasing intrauterine device and estrogen-progestin contraceptives in patients who desire to retain fertility. Complex hyperplasia is also expected to regress (56%) when atypia is not present. Atypical hyperplasia has a 36% progression rate, and even with progestins, 27% have been reported to progress, but it is uncertain if this is coexistent cancer that is eventually identified. Fifty-five percent of atypical hyperplasia is expected to regress with progestin therapy. A study among Finnish women notes that a treatment regimen including continuous progestin rather than cyclical (10–14 days/month) progestin appears to reduce risk of endometrial cancer in a postmenopausal population. It is assumed that unless the estrogen stimulation ceases, the progestin therapy may need to be lifelong. Alternatively, a hysterectomy is necessary to prevent the development of endometrioid adenocarcinoma ( Fig. 4.4 ) or type I cancer (estrogen dependent). This estrogen-induced cancer is the most common histologic type and the least aggressive form of uterine malignancy and should be preventable, as well as successfully treated when detected.
The EIN system developed by International Endometrial Collaborative Group classifies endometrial precursors based on their clonal origin, noninvasive growth patterns, and risk of concurrent carcinoma. For a diagnosis of EIN to be made, the following criteria must be met: area of glands exceeds the stroma, cytology differs between the crowded focus and the normal background endometrium, the size of the lesion exceeds 1 mm and benign pathology with overlapping features such as polyps or effects of exogenous estrogen can be eliminated. This system is not universally adopted at this time, but there is some evidence that the clinical outcome prediction and the interobserver reproducibility using EIN may be improved over the 1994 WHO schema.
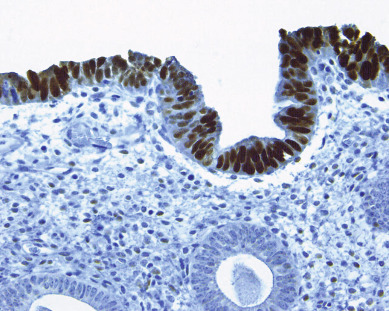
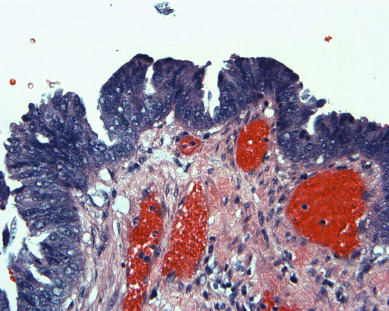
Endometrial carcinomas of the serous papillary variety arise in a background of atrophic endometrium. These cancers are not associated with excess estrogen and have been classified as type II (estrogen independent) endometrial cancers. Serous papillary adenocarcinoma is less likely to be identified at an early stage compared with endometrioid histologic types. These lesions are typically associated with mutations in the p53 tumor suppressor gene that results in accumulation of inactive p53 protein in the cell ( Fig. 4.8 ). Unlike normal cells or most endometrioid cancers, immunostaining with antibodies to p53 will be positive in the majority of serous cancers. Endometrial hyperplasia is not a precursor to serous carcinoma. Rather, the precursor has been identified as endometrial intraepithelial carcinoma (EIC). Serous EIC ( Fig. 4.9 ) can even be multifocal with disease found in the ovaries, fallopian tubes, and peritoneal cavity when no invasive component is found in the uterus. Comprehensive surgical staging is recommended because of the difficulty in establishing the diagnosis of invasive cancer intraoperatively. Postoperative therapy can be recommended after adequate histologic evaluation.
Management Decisions for Atypical Endometrial Hyperplasia (Endometrial Intraepithelial Neoplasia)
The patient’s age, fertility plans and need for contraception, comorbidities, and personal preferences play a role in the management of these lesions. Hysterectomy is indicated when fertility is not desired and AEH is identified. AEH is commonly found with coexisting undiagnosed cancer already present in the uterus or progressing to endometrial cancer in untreated women. As previously discussed, the GOG reported that 43% of 306 women who had a community-based diagnosis of AEH on endometrial biopsy and proceeded without medical treatment to hysterectomy had endometrial cancer on final pathology. Although 63% (77 of 123) of the cancers identified were grade I lesions confined to the endometrium, 31% were myoinvasive, and 11% had deep myometrial invasion into the outer 50% of the myometrium. It is possible that future validation of a new EIN scoring system or another molecular marker will allow more precise predictability for the neoplastic potential and the coexistence of cancer before hysterectomy.
The complexity of the surgical management of AEH is underappreciated. Intraoperative decision making, using frozen section to determine the operative interventions, is never ideal, considering difficulty of the diagnosis and the variety of skill and expertise of the pathologist and gynecologists. It is to be expected that the postoperative diagnosis of cancer will be obtained in a substantial number of these cases. The recommendation is that the patient should be informed of the 20% to 45% risk of underlying malignancy, and hysterectomy is required for the final diagnosis. The patient should be counseled about the desire to keep her ovaries or have them removed based on her age, family history, and other medical conditions or comorbidities. Genetic counseling and testing for Lynch syndrome may also be reasonable as a factor to consider in this decision-making process. Vaginal hysterectomy may be all that is required, or laparoscopically assisted hysterectomy with bilateral salpingo-oophorectomy can be considered. For the vast majority of patients with AEH, lymphadenectomy and complete surgical staging results in overtreatment and increased surgical risk. Endometrial cancers associated with AEH are usually low-grade, early stage lesions with a low risk of lymphatic metastasis. The risk of a high-risk uterine cancer in women with a biopsy of AEH ranges from 5% to 7%. In the event that high-grade cancer or deep myometrial invasion is found, reoperation may be offered for comprehensive surgical staging and removal of retained ovaries. Staging can usually be accomplished laparoscopically. This should be necessary in approximately 15% of the cases when cancer is identified and depends on the entire health history of the individual. Staging based on intraoperative assessment of myometrial invasion and grade of tumor is an appropriate strategy for institutions experienced in this approach. Supracervical hysterectomy, morcellation, and endometrial ablation are unacceptable for treatment of AEH because of the high prevalence of occult endometrial cancer in these patients. Women who desire childbearing, refuse hysterectomy, or have medical conditions that make hysterectomy an undesirable first choice can be treated hormonally. Megestrol 160 mg/day, in divided doses, has been the drug of choice with acceptable results even in the face of complex endometrial hyperplasia with atypia. It is unclear whether the treatment should be continuous or cyclic, but there are theoretical advantages to the endometrial shedding provided by the progesterone withdrawal bleed. The endometrium needs to be reevaluated histologically, by office biopsy or D&C, at approximately 3-month intervals for at least 1 year. Lifelong prevention strategies must be emphasized with the patient. The pathologist finds the evaluation of the endometrial sampling more straightforward if it is not complicated by exogenous hormonal influences. For this reason, it is best to withdraw the patient from the progestogen for 7 to 14 days to allow withdrawal bleeding before endometrial biopsies. The biopsy or D&C should be scheduled for after the withdrawal bleeding ceases.
In 2012, a systematic review identified 391 subjects from 45 studies with hyperplasia or grade 1 endometrial cancer who desired fertility and were treated with progestins. Seventy-eight percent of patients demonstrated a response to therapy with 53% of patients demonstrating a durable, complete response. The complete response rate was significantly higher for those with hyperplasia at 65% than for those women with carcinoma (48%). The American Congress of Obstetricians and Gynecologists (ACOG) Practice Bulletin on endometrial cancer includes this general guideline: The new Endometrial Intraepithelial Neoplasia ACOG Practice Bulletin (May 2015) offers multiple options for women with contraindications for hysterectomy, including medroxyprogesterone acetate, depot medroxyprogesterone, micronized vaginal progesterone, megestrol acetate, or levonorgestrel intrauterine system.
Women with AEH and endometrial cancer who desire to maintain their fertility may be treated with progestin therapy. After therapy, they should undergo serial complete intrauterine evaluation approximately every 3 months to document response. Hysterectomy should be recommended for women who do not desire fertility.
Local therapy of the endometrium with a progestin-containing intrauterine device (Mirena) is encouraging. In a recent multicenter randomized trial in Norway, 170 women with endometrial hyperplasia were randomized to one of three treatment arms: levonorgestrel intrauterine system (LNG-IUS), oral MPA 10 mg administered 10 days per cycle, or continuous oral MPA 10 mg/day for 6 months. At the end of 6 months, women in all treatment regimens showed significant therapeutic response, but the highest numbers of responders were noted in the LNG-IUS group (100%) and the continuous MPA group (96%) compared with the cyclical MPA group (69%). Gallos et al. reported a 12-year comparative cohort study of 344 patients with endometrial hyperplasia who received conservative management with progestins for complex or AEH because of desire for fertility or who were medically unsuitable for surgery. For the group of women treated with LNG-IUS ( n = 250), 94.8% achieved regression of hyperplasia compared with 84% of women treated with oral progestins. Of note, the patients with a body mass index of 35 or greater were most likely to have a relapse of hyperplasia or fail to regress to a normal endometrium. In conclusion, management decisions in women with AEH are complex and require an understanding of the risk of invasive endometrial adenocarcinoma, the reproductive desires of the patient and her comorbidities, and the risks for surgical management. Ideally, minimally invasive techniques can be used to complete hysterectomy with bilateral salpingo-oophorectomy. The finding of adenocarcinoma in the uterus requires consultation with a gynecologic oncologist to determine whether observation, reoperation for surgical staging, or adjuvant therapy based on uterine factors should be considered.
Management of Endometrial Hyperplasia Without Atypia
The diagnosis of simple or complex hyperplasia without atypia requires hormonal management and is not an indication for hysterectomy. These lesions are generally reversible with progestogen (synthetic progestin or progesterone). The classification of progestogens is seen in Table 4.2 . The initial approach is generally to treat in a cyclic fashion with the progestin given for 14 days of the month to deliver predictable cyclic withdrawal menses. Women, including teenagers who are premenopausal and sexually active, are usually best treated with a regimen that provides contraception such as Depo-Provera or oral contraceptives. Women who want to conceive are likely to be anovulatory and require ovulation induction agents after withdrawal bleeding is produced with a progestogen. Reassurance can be obtained from a reduction of menstrual flow and a bleeding pattern that is appropriately timed with the cyclic therapy. It is extremely important to counsel anovulatory women about the lifelong risk of endometrial cancer and methods for surveillance and prevention. Twenty-five percent of endometrial cancers occur in the premenopausal age range, and 5% are in women 40 years of age and younger. These cancers may be part of the polycystic ovarian syndrome or the anovulatory transition before menopause, and are all theoretically preventable with proper counseling and active management. Premenopausal endometrial cancer patients should also be counseled about the possibility of Lynch syndrome. A family history should be taken, and if endometrial and colon cancers are frequent in the family, the patient should be referred to genetic counseling. Colonoscopy is often recommended to endometrial cancer patients because of the association with colon cancer. There is a dramatic rise in the incidence rate of endometrial hyperplasia and endometrial cancer at 45 years of age and peaking at 65 years of age. This is likely because of the combined effects of estrone (estrogen) production in the peripheral adipose tissue, particularly in obese women, and to the absence of progesterone that is caused by the loss of ovulatory function in menopause.
| Hormonal Agent | Dosage and Length |
|---|---|
| Medroxyprogesterone acetate | 10–20 mg/day or cyclic 12–14 days per month |
| Depot medroxyprogesterone | 150 mg intramuscularly every 3 months |
| Micronized vaginal progesterone | 100–200 mg/d or cyclic 12–14 days per month |
| Megestrol acetate | 40–200 mg/day |
| Levonorgestrel intrauterine system | 52 mg in steroid reservoir over 5 years |
Prevention of Endometrial Cancer
Endometrial stimulation by estrogens unopposed by progestins leads to endometrial hyperplastic conditions in a dose- and time-dependent manner. Experimental evidence has been obtained from prospective clinical trials conducted to identify the appropriate doses and schedules of hormone replacement therapies. Kurman reported a randomized trial of 1176 postmenopausal women receiving 1 mg of estradiol orally and either placebo or various doses of norethindrone acetate. Women treated with estradiol alone (247 evaluable participants) for 12 months had a 12.2% rate of simple hyperplasia without atypia, 1.6% had complex hyperplasia without atypia, and 0.8% had complex atypical hyperplasia. Norethindrone acetate at all doses used in this trial nearly eliminated that risk. The North American Menopause Society support the addition of a progestogen whenever estrogen is prescribed for menopausal symptoms in women with an intact uterus for the prevention of estrogen-induced hyperplasia and adenocarcinoma. Unfortunately, progestogen has undesirable side effects, and women have been known to discontinue this component of their prescribed hormone replacement (see Table 4.2 ).
The prevention of endometrial cancer has been complicated by the publication and early closure of the estrogen plus progestin (E + P) component of the Women’s Health Initiative (WHI). This randomized placebo-controlled clinical trial evaluated conjugated equine estrogens (CEEs), 0.625 mg, plus MPA, 2.5 mg orally per day (hormone replacement therapy [HRT]). This trial enrolled 16,608 women with a range of 50 to 79 years of age for an average of 5.2 years to determine the primary outcomes of coronary heart disease (CHD), invasive breast cancer, and a global index (including stroke, pulmonary embolism [PE], endometrial cancer, colorectal cancer, hip fracture, and death from other causes). In May 2002, the data safety monitoring committee recommended premature closure of the trial because of the adverse outcomes of breast cancer and cardiovascular complications, including CHD, stroke, and PE. Of note, the overall mortality rate was not affected. The absolute excess risk per 10,000 person-years attributable to HRT was seven CHD events, eight strokes, eight PE, eight invasive breast cancers, and a reduction of five hip fractures. These risks need to be reviewed with women choosing HRT.
The WHI trial with CEE alone versus placebo had a very different outcome. There was a nonsignificant reduction in breast cancer risk ( P = 0.06), and the CHD risk was unaffected (hazards ratio [HR], 0.91; CI 0.75–1.12), strokes were increased (HR, 1.39; CI 1.10–1.77), and hip fractures were reduced (HR, 0.61; CI 0.41–0.91). This places women with intact uteruses and their physicians in a difficult situation of balancing the risks. Women with uteruses can decide to accept the potential risks of the addition of a progestin or may choose to take unopposed estrogen and follow the endometrium yearly with transvaginal ultrasonography or endometrial biopsy. Unopposed estrogen use gives a woman a relative risk (RR) of endometrial cancer of three times the general population for less than 5 years of use and RR of 10 after 10 years of use. The risk decreases but remains elevated after discontinuing the drug. Alternatively, other progestins and progesterone formulations are available that may carry a different but as yet unknown risk of breast cancer. Micronized progesterone is available as oral 100- or 200-mg capsules (Prometrium) and has been approved by the Food and Drug Administration (FDA) for use in combination with estrogen for relief of menopausal symptoms. The dosage of 200 mg for 12 days per month in a cyclic fashion causes withdrawal bleeding in a predictable manner. The regimen of CEE 0.625 mg/day with oral micronized progesterone at a dose of 200 mg/day for 12 days per month failed to see any increase in endometrial hyperplasia with 3 years of follow-up. A vaginal progesterone gel is available that is only FDA approved for infertility use but has been studied in small numbers of menopausal women. An additional provocative alternative is to use the progestin-containing intrauterine system (Mirena is FDA approved for contraceptive use only) and unopposed estrogen, which has been studied prospectively in menopausal women. These women were only followed for 1 year, and they were able to convert proliferative endometrium to atrophic endometrium while using continuous estradiol 50 mcg/day via the transdermal route.
The official position statement of the North American Menopause Society is that a progestogen should be added to estrogen therapy for all postmenopausal women with intact uteruses. The type, route, or regimen can be individualized to minimize side effects while providing adequate endometrial protection.
Benefits and Risks of Estrogen Replacement Therapy
Quality of Life, Vasomotor Symptoms, and Sexual Function
The WHO and the Stages of Reproductive Aging Workshop (STRAW) working group define menopause as the permanent cessation of menstrual periods that occurs naturally or is induced by surgery, chemotherapy, or radiation. Menopausal transition is the time of an increase in follicle-stimulating hormone and increased variability in cycle length. Postmenopause begins at the time of the final menstrual period but is not recognized until 12 months of amenorrhea has occurred. The National Institutes of Health in the “State of the Science Conference Statement: Management of Menopause-Related Symptoms” defines menopausal symptoms as vasomotor symptoms, vaginal dryness and painful intercourse, and sleep disturbance. Estrogen is the most consistently effective therapy for vasomotor symptoms, vaginal dryness, sleep disturbances, and improved quality of life, and a subset of women may find an improved mood with estrogen therapy. Testosterone has been demonstrated to improve libido, especially in women who have undergone oophorectomy; however, the long-term risks have not been studied. Antidepressants, clonidine, and gabapentin have been studied in treatment of hot flashes with some efficacy.
Symptom control is the most common reason for initiating estrogen therapy and remains undisputed as the most appropriate indication for prescribing estrogen at the time of natural or surgical menopause. The epidemiology community recommendations are that the patient should be told that hormone replacement should be for symptom control only, prescribed at the lowest effective dose, and for the shortest duration of use possible. Women should consider tapering off after 5 years of use to avoid the adverse events of thromboembolic disorders, breast cancer, stroke, and cardiovascular events (myocardial infarction, death). Women must be active participants in the decision-making process regarding estrogen use or estrogen plus progestogen use. They must experience the symptoms, recognize the reasons they are choosing to use these agents, and actively make the decision that the benefit is worth the small risk. Their own social situation or individual risk profile may influence their personal decisions.
The WHI reported the symptoms manifested by 8405 women between 8 to 12 months from the date the HRT participants were asked to discontinue CEE plus medroxyprogesterone acetate. These women were mailed a survey, and 21.2% responded that they had moderate-to-severe vasomotor symptoms, which was significantly different (CI 4.92–6.89) compared with placebo. They also reported pain and stiffness (Adjusted odds ratio [AOR] 2.16), and these symptoms were reported by more than 10% of respondents. Other withdrawal effects were feeling tired, difficulty sleeping, and a feeling of bloating or gas. Depression was also significantly increased after withdrawal of hormone replacement ( P <0.001).
The WHI documented the beneficial effects experienced from HRT in women with uteruses. Relief of hot flashes (85.7%), night sweats (77.6%), vaginal or genital dryness (74.1%), and joint pain or stiffness (49.1%) compared with placebo effects ranged from 57.7% to 38.4%, respectively. Women taking HRT were more likely to complain of breast tenderness (9.3%) and vaginal bleeding (51%) than those on placebo (2.4% vs. 5%, respectively).
In conclusion, the data summarized in the WHI are not generalizable to 45- to 55-year-old women with menopausal symptoms. These women must prioritize their own situations and health risks and quality of life benefits. Most will find the best symptom relief from estrogen use. Alternatives to estrogen include progestogens, antidepressants (venlafaxine, paroxetine, fluoxetine), clonidine, and the anticonvulsant gabapentin. Over-the-counter remedies frequently used include isoflavones, black cohosh, and vitamin E. None of the alternatives resolve the entire menopausal syndrome, including sexual dysfunction.
Estradiol may also be delivered by a silicone ring placed in the vagina (Estring, Femring). The Estring (Pfizer US Pharmaceuticals) contains 2 mg of estradiol and on average 0.02 mg/day is released over a 90-day period. Studies have demonstrated a median plasma estradiol increase from 4.5 pmol/L before insertion to 12.5 pmol/L at steady state. The comparison with a 0.05 mg Estraderm patch was 10-fold greater serum concentrations of estradiol in users of the patch. A study of endometrial thickness in 60 postmenopausal women over a 12-month period showed a thickness of 2.8 mm before Estring and 2.6 mm thickness after 12 months of use. This is reassuring to women unable to tolerate progestins. Another randomized controlled study (RCT) demonstrated a decrease in urinary tract infections with the use of Estring. The use of Estring may be preferred for women wanting local therapy for vaginal dryness symptoms and an inability to take progestins or a history of breast cancer. Femring is available when an increase in vaginal dose is desired.
Estrogen alone does not always resolve the sexual dysfunction complaints after bilateral salpingo-oophorectomy. Testosterone has been demonstrated in clinical trials to help with decreased sexual desire, arousal, and orgasmic response, but at present, there is no FDA approval for libido in women. A recent study evaluated the efficacy and safety of testosterone treatment in 814 postmenopausal women with hypoactive sexual desire not receiving estrogen therapy. Patients were randomized to receive testosterone by patch (150 or 300 mcg/day) or placebo. At 24 weeks, efficacy was measured, and the group of women receiving 300 mcg/day of testosterone reported significantly greater sexual satisfaction compared with the placebo group and the group treated with 150 mcg/day of testosterone. The rate of adverse events related to androgens, specifically unwanted hair growth, was also highest in this group. Furthermore, breast cancer was diagnosed in four women treated with testosterone compared with none of the women who received placebo. The long-term effects of testosterone, including effects on the breasts, remain unclear. The FDA has recently approved flibanserin to treat acquired, generalized hyposexual desire disorder in premenopausal but not postmenopausal women.
Osteoporosis
Osteoporosis is a disease characterized by low bone mass and microarchitectural deterioration of bone tissue, which leads to increased bone fragility and increased fracture risk. Low bone mass is related to a low peak bone mass (mainly under genetic influence) and to decrease in bone mass, which occurs after menopause and with aging. Menopause induces an accelerated bone loss within 5 to 8 years followed by a linear rate of bone loss. Bone density readings are standardized to healthy women of similar age. Osteopenia is defined as bone loss of greater than 1 standard deviation (SD) from the mean, and osteoporosis is bone loss greater than 2 SDs from the mean.
Osteoporosis prevention strategies should begin before 40 years of age because of a decline from peak bone mass. An even more dramatic loss of bone occurs at menopause with the loss of estrogen support of osteoblastic activity. Women should take active measures to protect their bones by regular weight-bearing exercise (at least 30 minutes three times a week), calcium supplementation (1500 mg elemental calcium for postmenopausal women), and adequate vitamin D supplementation (800–1200 IU/day). At menopause, a baseline bone density is recommended and then repeated every other year unless osteopenia or osteoporosis is detected. Estrogen replacement therapy (ERT) has been the traditional main strategy to prevent this deterioration in bone strength. A number of medications are approved by the FDA for the prevention or treatment of osteoporosis. Bisphosphonates such as alendronate (Fosamax), risedronate (Actonel), and ibandronate (Boniva) act by preventing bone resorption by blocking the action of osteoclasts. Gastrointestinal side effects (reflex, esophagitis, esophageal ulcers) have been a primary concern for patients taking oral bisphosphonates. These drugs should be taken first thing in the morning on an empty stomach with a full 8-oz glass of water. The patient should then wait a full hour before eating or taking any other medications. Lying down or eating before the recommended time increases risk for gastrointestinal distress. There are also theoretical concerns that prolonged therapy with bisphosphonates leads to oversuppression of bone remodeling, which allows microdamage to accumulate and increase skeletal fragility. Microscopic crack frequency is increased in animals treated with high doses of bisphosphonates, although this has yet to be demonstrated in biopsies taken from postmenopausal women treated with long-term use. SERMs such as raloxifene (Evista) bind with high affinity to estrogen receptors and promote estrogen-like activity on bone. Unfortunately, raloxifene has the same thromboembolic risk of estrogen without the benefit of menopausal symptom relief. Drugs approved for treatment only include calcitonin (Miacalcin), which is administered by nasal spray, and teriparatide (Forteo), which is administered through daily subcutaneous injections. Both of these pharmacologic agents serve to increase bone formation.
Two anatomic areas are of interest in the bone remodeling process. The first is the axial skeleton, which is composed primarily of trabecular bone. The second is the appendicular skeleton, which is composed primarily of cortical bone. The remodeling cycle is the same in both types of bone. However, because of the greater surface area, approximately 40% of trabecular bone, as opposed to 10% of the cortical bone, is in “turnover” each year. The osteoclast is responsible for the resorption of old bone, which results in the formation of a resorption cavity. Osteoblasts are then attracted to the cavity, where they secrete osteoid, which is primarily type I collagen. The collagen is mineralized mainly with calcium, thus producing new bone with an appropriate mechanical strength. Under normal circumstances, the amount of bone removed is replaced with fresh bone; however, this process can become uncoupled if osteoclasts remove more bone than can normally be replaced by osteoblasts, resulting in net loss of bone mass. In menopausal women, accelerated bone loss is associated with high bone turnover rate and increased osteoclast activity. Estrogen may inhibit osteoclast activity and increase proliferation of osteoblasts as well as collagen production.
The beneficial effects of estrogen in preventing or treating postmenopausal osteoporosis are well recognized. At the same time, there is growing recognition that osteoporosis is a major public health problem in the United States. It is estimated that approximately 8 million women in the United States have osteoporosis and another 14 million are at risk because they have low bone mass. Osteoporosis causes more than 1.5 million fractures each year, including 300,000 hip, 700,000 vertebral, and 200,000 wrist fractures. These fractures are seen mainly in women. For a 50-year-old woman, the lifetime risk of fracture of any of the three is 40%. Traditional risk factors for osteoporosis include estrogen deficiency; fair complexion; high caffeine intake; low calcium intake; small, thin build; smoking; inadequate physical activities; and multiple disease processes and drugs. The acute and long-term medical care expenses associated with these fractures cost the nation an estimated $17 billion in 2005. The cumulative cost over the next 2 decades is estimated to be $474 billion. An excess mortality rate associated with hip fracture is 12% to 20% during the first year after the injury. Fewer than half of patients with hip fractures ever return to their prefracture activity level. Many require assistance and have lost their independence and require long-term domiciliary care. Vertebral fractures may be asymptomatic in 50%, but successive fractures can lead to loss of height, kyphotic distortion of posture, and chronic back pain. Approximately 10% of patients with hip fractures die of surgical complications within 6 months of fracture. Approximately 25% of all white women older than 60 years of age have spinal compression fractures resulting from osteoporosis. The risk of hip fracture is 20% by the age of 90 years, and hip fractures are about 2.5 times more common in women than in men. An increased rate of loss of both cortical and cancellous bone is associated with menopause. In a follow-up study of 82 postmenopausal women 5 to 10 years after their first examination, Meema and coworkers concluded that (1) as a group, menopausal women lost bone, and the beginning of this loss is less related to age than to loss of ovarian function; (2) the rate of loss was not significantly correlated with age; and (3) the bone loss was prevented by estrogen administration (ie, 0.625 mg of conjugated estrogens).
Nachtigall and associates conducted a 10-year double-blind prospective study to evaluate the effects of ERT. They took a sample population of 84 pairs of randomly chosen postmenopausal patients who were matched for age and diagnosis. Half of the patients received conjugated estrogens and cyclic progesterone, and the other half received a placebo. The estrogen-treated patients whose therapy was started within 3 years of menopause showed improvement or no increase in osteoporosis. The patients in the control group demonstrated an increase in osteoporosis. A subsequent report by the same authors showed that there was no statistically significant difference in the incidence of thrombophlebitis, myocardial infarction, or uterine cancer in the two groups. Indeed, there was a lower incidence of breast cancer in the treated group. Estrogen-treated patients did show a higher incidence of cholelithiasis. The low number of cases precludes drawing any real conclusions from the data on diseases of low frequency. The study excludes a high incidence of complications from estrogens.
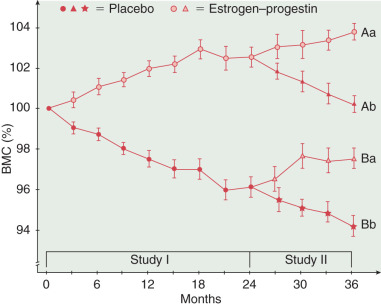
In another crossover study comparing the effects of estrogen–progestin therapy with those of placebo, bone mineral content increased during the 3 years of combination hormone therapy but continued to decrease in the placebo-treated group ( Fig. 4.10 ). When the placebo was given to some of the estrogen-progestin group, bone density decreased, but the placebo-treated women had an increase in bone mineral content after being given estrogen–progestin therapy. Other factors are also important in preventing osteoporosis, such as adequate dietary calcium, exercise, and vitamin D. Many case control studies have noted considerable protection from hip fracture with HRT ( Table 4.3 ).
| Authors | Relative Risk |
|---|---|
| Hutchinson et al. | 0.70 |
| Weiss et al. | 0.43–0.77 |
| Paganini-Hill et al. | 0.42 |
| Kreiger et al. | 0.50 |
| Kiel et al. | 0.34–0.65 |
| Naesser et al. | 0.79 |
| Kanis et al. | 0.55 |
Colorectal Cancer
Colorectal cancer is the third most common cancer and the third leading cause of cancer deaths in US women. Several epidemiologic studies have noted that HRT may reduce the risk of colorectal cancers. The WHI demonstrated an HR of 0.63, demonstrating slight protection from colorectal cancer in their E + P arm but not in the estrogen alone study (HR, 1.08). There appear to be some biologic reasons why estrogen may be protective. Bile acids are thought to initiate or promote malignant changes in the epithelium of the colon. Exogenous estrogen decreases secondary bile acid production. Estrogen also decreases serum levels of insulin-like growth factor-1, which is an important mitogen associated with colorectal cancer. Both case-control and prospective studies have been reported. Kampman and associates reported the largest case-controlled study with 815 colon cancer and 1019 population base controls in postmenopausal women. After adjusting for multiple risk factors, they noted an 18% decrease in risk of colon cancer in HRT users compared with the nonusers (RR, 0.82; CI 0.67–0.99). In the large study of the American Cancer Society, 897 deaths from colon cancer were identified during 7 years of follow-up of 422,373 postmenopausal women. The authors reported a 29% decrease in risk of colon cancer deaths in hormone users compared with nonusers (R, 0.71; CI 0.61–0.83). In the review and meta-analysis by Grodstein and colleagues, 18 studies were evaluated. They found a 20% decrease of colon cancer in ERT users compared with the nonusers (RR, 0.8; CI 0.72–0.92) and a 19% decrease in rectal cancer (RR, 0.79; CI 0.72–0.92). Ten studies provided data on timing of hormone use. Current users had an RR of 0.66 (CI 0.59–0.74) compared with that of the nonusers. In the Nurses’ Health Study, current users noted an RR of 0.65; in past users, the RR was 0.84 (nonsignificant). Among past users, the decreased risk continued during the first 4 years after quitting ERT; however, 5 years or more after stopping ERT, the risk was similar to that of nonusers (RR, 0.92; CI 0.70–1.21). The effect of ERT on colon cancer risk was evaluated in the California Teachers Study among 56,864 postmenopausal women. There was noted to be significantly lower risk for colon cancer in women who had used ERT within the past 15 years than women that had never used hormones (RR, 0.64; CI 0.51–0.80). Five studies evaluated the duration of current use and found similar apparent protection for all women currently on ERT, regardless of duration. Four studies evaluated ERT and colorectal adenoma. Most studies noted a significantly decreased risk of development of adenomas in women on ERT. This decrease did not appear to be caused by increased surveillance (sigmoidoscopy) in the ERT users. In several, but not all, studies, there was a suggestion that colorectal cancers increased in women who were taking tamoxifen.
Most recently, extended follow-up data from the WHI was published that reviewed both incidence and mortality of colorectal cancer in women treated with E + P. This analysis revealed that, although fewer colorectal cancers were diagnosed in the E + P group, the patients more commonly had positive lymph nodes and advanced stage at diagnosis, translating into more deaths. So, although the incidence of colorectal cancers was lower, the mortality rate was greater. These data address the importance of not only identifying the incidence of colorectal cancer but also the influence of tumor characteristics, stage, and mortality rate. Overall, the findings do not suggest a clinically meaningful benefit in reducing mortality from colorectal cancer.
Cardiovascular Disease
Cardiovascular morbidity (ie, heart disease and stroke) constitutes the most frequent cause of death among women in the United States. It is estimated to account for approximately 292,188 deaths in the United States in 2009—that is, one in every four female deaths. Cardiovascular risks factors can be found, and potentially modulated, in women. High cholesterol levels are present in 50% of women, and high blood pressure is observed in 32% of all women, with 45% of black women having high blood pressure. The prevalence of cardiovascular diseases increases dramatically with age, starting at a low level of 17.6% for women 35 to 44 years of age, 36.6% for women 45 to 55 years of age, 56.5% for women 55 to 64 years of age, and 75% for women 65 to 74 years of age. The possible effects of estrogen and progestin on the incidence of cardiovascular disease in women is the subject of ongoing research. Most of the cohort studies suggest that ERT effectively diminishes the risk of cardiovascular disease in women who are 50 to 60 years of age, but protection decreases with age ( Table 4.4 ). Unfortunately, the studies (WHI, Heart and Estrogen/Progestin Replacement Study [HERS]) reveal that initiating estrogen therapy in women with established vascular disease (mean ages of 63–67 years) will not prevent cardiovascular events or deaths. These studies are unable to be generalized to estrogen use in the younger perimenopausal age groups, and the cohort and epidemiologic studies reveal some benefit, but use of progestin consistently decreases benefit.
| Authors | Year | Relative Risk |
|---|---|---|
| Bush et al. | 1983 | 0.4 |
| Bush et al. | 1987 | 0.3 |
| Wilson et al. | 1985 | 1.3–32 * |
| Stampfer et al. | 1985 | 0.3 |
| Colditz et al. | 1987 | 0.42 |
Prevention of cardiovascular disease includes smoking cessation, blood pressure control, weight control, exercise, and blood sugar and cholesterol control. Blood cholesterol level is the main risk factor for cardiovascular disease in postmenopausal women. A high positive correlation has been found between levels of low-density lipoprotein (LDL) and coronary morbidity. Conversely, the level of high-density lipoprotein (HDL) shows a high negative correlation with the probability of coronary disease and with the extent of damage to the coronary vessels. Matthews has shown the beneficial effects of ERT on LDL and HDL levels. In a prospective study, he showed clearly a corresponding rise in HDLs and a decrease in LDLs in postmenopausal patients treated with ERT. The association of increased HDL and decreased LDL levels with reduced risk of cardiovascular disease is well documented for both men and women.
Lobo reported on the impact of different dosages of MPA on metabolism and hemostasis in postmenopausal women treated with conjugated estrogens. In this prospective, double-blind study, 525 women were randomized to five treatment regimens at 26 sites in the United States and Europe. All participants received 0.625 mg of conjugated estrogens daily for up to 13 cycles; four groups also received MPA, either 2.5 or 5 mg/day continuously or 5 or 10 mg/day for the last 14 days of each cycle. Effects on lipid and carbohydrate metabolism and coagulation were evaluated.
All of the treatment groups experienced increases in HDL cholesterol (HDL-C), the HDL2-C subfraction, and apolipoprotein A-1 compared with baseline; however, at each time point, the increases from baseline in the conjugated estrogens–only group were significantly greater than were those for the conjugated estrogens–MPA groups. Additionally, all treatment groups had significant decreases in LDL cholesterol (LDL-C) and apolipoprotein B. Decreases from baseline in total cholesterol (TC) levels were significantly greater than were those in the conjugated estrogens–only group. HDL3-C levels increased significantly from baseline in the conjugated estrogens–only group but not in the other treatment groups. Triglyceride levels increased significantly in all groups. The mean increase in the MPA-treated groups was less than that in the conjugated estrogens–only treatment group. Although MPA modified some of the effects of conjugated estrogens on lipid metabolism, the direction of changes in lipid parameters was unaltered.
Additional data on the conjugated estrogens regimen were reported in the results of the Postmenopausal Estrogen/Progestin Intervention (PEPI) trial. In this 3-year, randomized, double-blind, placebo-controlled trial, the investigators evaluated differences in selected heart disease risk factors among 875 postmenopausal women who received placebo, conjugated estrogens, or one of three conjugated estrogens–progestin regimens. One of the regimens used in this study was identical to that present in conjugated estrogens.
The investigators reported that after 3 years of therapy, women taking the conjugated estrogens regimen experienced significant increases in HDL-C levels compared with those taking the placebo. However, the increases were significantly less than those experienced by women taking conjugated estrogens alone. Compared with the placebo, LDL-C values were significantly decreased in women taking the conjugated estrogens, with no significant difference between groups. TC levels decreased significantly among women taking the conjugated estrogens regimen compared with those taking the placebo. Significant increases in serum triglyceride levels occurred in all treatment groups compared with the placebo group. There were no significant changes in blood pressure, fasting insulin levels, or 2-hour insulin levels between treatment groups. Treatment groups had significant increases in 2-hour glucose levels and significant decreases in fasting glucose levels compared with the placebo group. In paired comparisons, women in the placebo group had greater increases in fibrinogen than did women in the active treatment groups; no significant differences were present among the active treatment groups. Among their findings, the investigators concluded that “… oral estrogen taken alone or with MPA … is associated with improved lipoprotein and lower fibrinogen levels compared with placebo and the magnitude of these differences is likely to be clinically significant.”
Multiple studies have evaluated HRT and CHD. In 1998, an overview by Barrett-Connor and Grady included 25 studies. Overall, the RR of CHD for women who took ERT compared with those who never took ERT was 0.70 (CI 0.65–0.75). When HRT was evaluated, the RR was 0.66 (0.53–0.84) compared with the nonusers. This analysis evaluated cohort case control and angiographic studies. The apparent benefit is limited mainly to current or recent use. These findings are consistent across diverse populations and various study designs.
The US National Health and Nutritional Examination Survey (NHANES-1) evaluated HRT and death from all cardiovascular disease in 1944 white women 55 years of age or older who were monitored for up to 16 years. After adjusting for risk factors of CHD, the risk of death from CHD was 0.66 (0.48–0.90) in the HRT users. The study by Ettinger and associates noted similar findings. In their patient population followed for 25 years, the death from any cause in HRT users compared with nonusers was 0.54, which was mainly due to a reduction in CHD (RR, 0.40) and other cardiovascular diseases (RR, 0.27).
Many studies, including the PEPI RCT, have noted that estrogen therapy in postmenopausal women decreased serum TC, LDL, and Lp(a) lipoproteins. HDL and triglyceride concentrations increase. The route of administration does affect serum lipids. Transdermal application has less effect on the serum lipid concentrations than the oral route. The type of progestins can also affect the serum lipids. Synthetic progestins tend to blunt the beneficial effects on the HDL but not decrease it below the baseline. Micronized progesterone does not decrease HDL compared with estrogen alone. Estrogen tends to decrease plasma fibrinogen concentrations as it does anticoagulant proteins, antithrombin III, and protein S. There also appears to be a decrease in the antifibronolytic protein plasminogen B activator inhibitor type I with an overall increased potential for fibrinolysis. In some reports, factor VII was also lowered with estrogen. The net effect on coagulation depends on the type of estrogen, dose, and duration of treatment.
The Global Consensus Statement on menopausal hormone therapy released a statement in 2013 recommending the use of estrogen therapy as the most effective treatment for vasomotor symptoms associated with menopause, and the benefits are more likely to outweigh the risks before age 60 years. Estrogen is effective and appropriate for the prevention of osteoporosis-related fractures in at risk women before age 60 years. They recommend the lowest dose and shortest treatment duration to achieve goals. This statement is based on the results of a round table meeting between representatives of the major international menopausal societies to reach consensus on core recommendations regarding HRT.
Two large trials (WHI and HERS) have now demonstrated that estrogen cannot protect against myocardial infarction or death in women with preexisting heart or vascular disease. In fact, the thromboembolic properties of estrogen likely increase risk when preexisting vascular lesions are present, predisposing women to stroke, myocardial infarction, and death. The WHI demonstrated in a trial of 16,608 women randomized to combined estrogen plus progestin (HRT) versus placebo at a mean age of 63.6 years. The absolute excess risk per 10,000 person-years attributable to HRT was seven CHD events, eight strokes, eight PEs, eight invasive breast cancers, and a reduction of five hip fractures. The WHI trial also had women without uterus treated with CEE alone versus placebo ( n = 10,739), and they had a very different outcome. There was a nonsignificant reduction in breast cancer risk ( P = 0.06), and CHD risk was unaffected (HR, 0.91; CI 0.75–1.12), strokes were increased (HR, 1.39; CI 1.10–1.77), and hip fractures were reduced (HR, 0.61; CI 0.41–0.91).
The HERS was designed to determine if HRT could reduce cardiovascular events in women with known coronary artery disease (CAD). A total of 2763 women with preexisting CAD and an average age of 67 years were enrolled and randomized to conjugated estrogen 0.625 mg plus MPA 2.5 mg/day versus placebo. After 4.1 years of follow-up, there was no significant difference in the primary outcomes of CHD events or in any secondary outcome. There was a time trend of higher risk of events in the first year of use and a decrease in events in years 3 through 5. HERS II was a longer term follow-up study of the same participants that could not substantiate any benefit of HRT with longer use. It has been speculated that the initial increase in risk of cardiovascular events with HRT might be due to prothrombotic effects of the hormonal therapy. There may be a beneficial effect on the inhibition of progression of atherosclerosis because of the effects on lipoprotein cholesterol; therefore, those who were doing well on the medication were not asked to discontinue it. The conclusion of HERS and HERS II was that postmenopausal hormone therapy should not be used to reduce the risk for CHD events in women with CHD.
Manson et al., for the WHI investigators, published the summary of CHD outcomes. This report also emphasized the increased risk of CHD events during the first year of use and recommended against prescribing HRT for the prevention of cardiovascular disease. The problems with these trials are that the participants were older than the usual women prescribed HRT, and they were recruited because of their increased risk of CAD events. This makes the results of HERS and WHI applicable to a similar population of high-risk women, and the conclusions may not be generalizable to younger women initiating HRT at the onset of menopausal symptoms. Prevention of CAD may have a different pathophysiology than the progression from CAD to death. The epidemiologic and cohort data are criticized for the likely enrollment of healthier and more active women and may also not represent all women of a similar age.
Cardiovascular risk reduction recommendations are smoking cessation, exercise, and weight control along with blood pressure and cholesterol management. Aspirin has recently been shown to lower the risk of stroke without affecting the risk of myocardial infarction or death from cardiovascular causes in most women. In this randomized trial of 39,876 healthy women 45 years of age or older, participants received 100 mg or aspirin or placebo on alternate days for 10 years and were monitored for cardiovascular events. There was a 17% reduction of strokes for the entire study population randomized to aspirin (RR, 0.83; CI 0.69–0.99; P = 0.04). There was benefit for prevention of myocardial infarction only in women 65 years of age and older at enrollment (RR, 0.66; CI 0.44; 0.97; P = 0.04). The conclusion of this study is that physicians should perform a careful risk assessment to determine who is likely to benefit from aspirin versus likely to be harmed by the increase of gastrointestinal hemorrhage requiring transfusion ( P = 0.05)
The 2013 ACOG committee recommended that HRT should not be used for the primary or secondary prevention of CHD, but rather women should be prescribed estrogen or estrogen plus a progestogen (when a uterus is present) at the onset of moderate or severe menopausal symptoms. The dose should be the lowest necessary to achieve the symptom control desired, and the duration of therapy should be 5 years or less. Discontinuation of this medication is not without side effects, and withdrawal symptoms may be minimized by a tapering schedule over a long period of time. It is accepted, however, that when a woman is on therapy, the risk of continuing such treatment is low, and the risk is greatest during the first year or two after initiating treatment, especially when preexisting vascular disease may already be present.
Estrogen Replacement Therapy for Endometrial and Breast Cancer Survivors
Estrogen Replacement Therapy for Endometrial Cancer Survivors
Historically, HRT has been contraindicated for patients who have been treated for cancer of the uterus. Although estrogen administration has not been shown to have adverse effects on patients who have been treated for endometrial cancer, many physicians continue to be reluctant to administer this medication to these women. No data in the literature substantiate the detrimental effects of estrogen in these patients. It is not unusual for women with endometrial cancer to have severe symptoms because of withdrawal of estrogen at the time of their diagnosis and surgical treatment.
The GOG conducted a prospective trial to evaluate the potential risk of estrogen replacement in women after treatment of endometrial adenocarcinoma. This randomized clinical trial of ERT (CEE 0.625 mg orally per day, Premarin) compared with placebo in women treated for stage I and II endometrial cancer closed after the WHI was halted prematurely in 2002 because of decrease in accrual and lack of feasibility. Before study closure, 1236 eligible women were randomized, and the median follow-up was 35.7 months. The median age at endometrial cancer diagnosis was 57 years (range, 26–91 years). Compliance with medication for the entire treatment period was 41%, and for those on the ERT arm, 71% knew to which arm they had been randomized. A summary of the results on the ERT arm demonstrated endometrial cancer recurrence in 14 patients (2.3%); eight patients (1.3%) developed a new malignancy, one of which was breast cancer. There were 26 deaths (4.2%) on the estrogen arm; five were due to endometrial cancer. An important finding is that women with stage I and II endometrial cancer are more likely to die of causes unrelated to the cancer diagnosis; therefore, other health issues must take priority when planning for preventive health maintenance ( Table 4.5 ). Women on the placebo arm of this study suffered 19 deaths (3.1% not statistically different), and four were due to endometrial cancer. Ten placebo patients developed a new malignancy (1.6%), three of which were breast cancer. Although there were no significant differences in recurrence between the ERT and placebo arms for all comers, further investigation suggested that racial disparities in outcome may exist. With a median follow-up of 3 years, recurrent disease was noted in 5 of 56 black patients on the ERT arm compared with only 8 of 521 white patients. The RR of recurrence among blacks in the ERT group was 11.2 (95% CI, 2.86–43.59; P = 0.0005) after adjustment for age, body mass index, and tumor grade.