Graph 1.1
Five-year relative survival trend in endometrial carcinoma based on SEER database
Table 1.1
Number of cases and number of deaths per 100,000 persons by race/ethnicity: endometrial carcinoma based on SEER database
Race/ethnicity | Number of cases | Number of deaths |
|---|---|---|
All races | 25.4 | 4.5 |
White | 26.0 | 4.1 |
Black | 24.6 | 7.9 |
Asian/Pacific Islander | 20.3 | 2.9 |
American Indian/Alaska Native | 21.3 | 3.6 |
Hispanic | 21.4 | 3.6 |
Non-Hispanic | 25.9 | 4.5 |
The Indian Council of Medical Research (ICMR) has published a 3-year report of population-based cancer registries (PBCRs) (2012–2014) from Bengaluru, India, in March 2016. On comparing the AARs (age-adjusted rates) per 100,000 persons, cancer of the corpus occupied the top three places in Chennai (6.0), Delhi (5.5), and Thiruvananthapuram districts (5.1). On analyzing the trends of endometrial carcinoma over time, PBCRs showed a significant increase in annual average of AARs for both three and five years in the metropolitan cities of Bengaluru, Chennai, Delhi and Mumbai.
Changing reproductive trends leading to prolonged estrogen exposure might be responsible for the increasing incidence of certain reproductive cancers in females. These include reduced age at menarche, delayed age at first pregnancy, less number of pregnancies, increased incidence of infertility, higher use of OCs/HRT, and increased number of ovulations. However, it is not practical to divert current reproductive practices to those of our ancestors, e.g., early first birth, having more children, etc. At the same time, healthy lifestyle and dietary habits should be promoted. Evolutional and designed changes in microanatomical and hormonal milieu of human physiology need to proceed while debating their social desirability.
According to Henderson et al. (1982), women with endometrial carcinoma typically exhibit signs of high estrogen effect and higher plasma estrogen levels as compared to controls. The association of obesity with endometrial carcinoma supports this hypothesis called as “estrogen excess hypothesis” [1]. Endometrial glandular proliferation is inhibited by endogenous progesterone in premenopausal women. Endometrial proliferation is markedly reduced in premenopausal women receiving a synthetic progestin and in untreated postmenopausal women [3].
Women’s Health Initiative (n = 16,608), a double-blind placebo-controlled trial, showed that after 5.6 years’ median intervention and 13 years’ median cumulative follow-up there were fewer endometrial carcinoma and statistically nonsignificant reduction in deaths from endometrial carcinoma in the combined hormone therapy compared with the placebo group [4].
A meta-analysis of 30 studies showed that the relative risk of ever users of unopposed estrogen therapy was 2.3 compared to nonusers, and it increased to 9.5 in users of 10 or more years [5].
The potential of anti-aromatase agents in management and the role of hormone receptors and immunohistochemical (IHC) markers in diagnosis as well as prognostication of endometrial carcinoma were also studied over a period of time.
Given the favorable responses to aromatase inhibitor therapy, as seen in women with endometrial carcinoma, these treatments may be of interest as preventive and adjunctive therapies for lesser proliferative lesions of the endometrium [6]. An overexpression of endometrial aromatase may underlie pathogenesis of endometrial polyps at least in a subset of cases [7].
Immunohistochemical analysis of endometrial carcinoma differentiating between various grades and histological types can be useful in identifying high-risk cases. Halperin et al found that the endometrioid G1–G2 cases showed increased immunoreactivity for ER, PR, and bcl-2 (85.7 %, 78.6 %, and 42.8 % respectively), and low expression of p53 (14.3 %) and HER-2/neu (14.3 %). In contrast, the serous papillary endometrial carcinoma cases showed immunonegativity for ER, PR, and bcl-2 and high immunoreactivity for p53 (81.8 %) and HER-2/neu (45.4 %). The endometrioid G3 cases demonstrated an intermediate immune profile characterized by immunonegativity for ER, PR, and HER-2/neu, low immunoreactivity for bcl-2 (7.1 %), and high expression of p53 (57.1 %) [8].
A review and meta-analysis report showed that in patients with endometrial carcinoma, higher level of ER and PR predicted favorable survival and increased level of HER2 was associated with poorer survival. All of the three hormone receptors had prognostic value for survival [9].
Precursor lesions like atypical endometrial hyperplasia (AEH) or endometrial intraepithelial carcinoma (EIC) frequently precede estrogen-related or serous endometrial carcinomas. However, prevalence of endometrial carcinoma is low (5 per 1000 women >45 years). Hence, standardized screening is not effective. At the same time, recognizing these precursor lesions and timely treatment will prevent these cancers. The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncology (SGO) do not recommend routine screening for uterine cancer. The American Cancer Society does recommend annual endometrial biopsies starting at age 35 for women known to have a risk for Lynch syndrome.
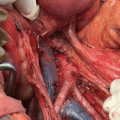
Timely assessment of symptomatic and high-risk patients is the key to correct diagnosis and management of endometrial carcinoma. When endometrial carcinoma was clinically staged (FIGO 1971), fractional dilatation and curettage was used to evaluate abnormal bleeding. This permitted the assessment of cervical tissue and endometrial tissue from all walls and surfaces of the uterus. Currently office endometrial biopsy has largely replaced D&C. The results of both methods correlate well and the accuracy to detect cancer is 91–99 % [10]. Hysteroscopy-guided biopsy is the standard practice used to evaluate abnormal uterine bleeding in many centers especially in postmenopausal women (Fig. 1.1). There is no substantial evidence to show that it improves the sensitivity to detect hyperplasia or cancers. Retrospective studies have suggested increased incidence of positive peritoneal cytology on hysterectomy after hysteroscopic evaluation. However, no prospective studies have been performed till date. Positive peritoneal cytology, independently, is not recognized as a stage-defining feature under the FIGO 2009 staging system [11].
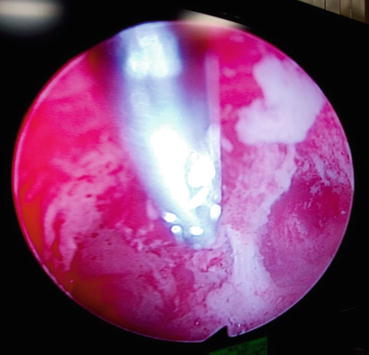

Fig. 1.1
Hysteroscopy-guided biopsy
There is very little role of preoperative imaging in patients with endometrial carcinoma, as surgery is essentially the same for stages 1, 2, and 3. Imaging studies have significant limitations in detecting nodal disease, which is microscopic in 90 % of cases [12]. Imaging studies may be more helpful in assessing extrauterine spread in serous and clear cell carcinomas, in determining operability to some extent, and in counseling young women opting for fertility-conserving surgery. In a small prospective series by Signorelli et al., a high negative predictive value for FDG PET/CT (93 %) was shown in high-risk endometrial carcinoma patients [13]. The GOG 233 trial is an ongoing prospective assessment of PET/CT in patients with endometrial and cervical cancer. Biomarker, CA 125, may be used to predict the presence of extrauterine disease. Ideally, serum biomarkers should be tested in endometrial tissue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree