Immune checkpoint inhibition has transformed cancer treatment and has improved long-term survival in patients with advanced malignancies. Antibodies that block cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD1) or its ligand (PD-L1) are now used routinely for cancer treatment. Autoimmune toxicities, known as immune-related adverse events (IRAEs), are seen with these therapies, including endocrine IRAEs. Hypophysitis, thyroid dysfunction, and insulin-dependent diabetes mellitus (IDDM) following immune checkpoint inhibition are seen with distinct clinical presentations.
Mechanism of Action of Anti-cytotoxic T-Lymphocyte Antigen 4 and Anti-programmed Death 1/or Its Ligand for Cancer Therapy
CTLA-4 is a glycoprotein expressed by T-cells and is the first immune checkpoint receptor to be targeted clinically. CTLA-4 controls the scale of early T-cell activation by inhibiting T-cell activity. CTLA-4 counteracts the activity of the T-cell costimulatory receptor, CD28. During an immune response, when the T-cell receptor (TCR) recognizes its cognate antigen, CD28 amplifies TCR signaling to activate T-cells. CD28 and CTLA4 share the same B7 ligands. CTLA-4 has a higher affinity for B7 and is thought to dampen T-cell activation by outcompeting CD28, as well as delivering inhibitory signals to the T cell. CTLA-4 binding inhibits interleukin-2 production and cell cycle progression of activated T-cells and prevents T-cell activation. , The inhibitory role of CTLA-4 in T-cell activation in check is shown by the lethal immune activation phenotype of CTLA-4-knockout mice. ,
Ipilimumab, a monoclonal antibody that binds to CTLA-4 and prevents B7 binding, is effective in the treatment of some malignancies ( Fig. 24.1 ). With B7 now accessible, CD28 binding upregulates T-cell activity. By blocking CTLA-4, activated T-cells proliferate and are persistently activated, which allows the targeting of previously poorly immunogenic tumor antigens to cancer cells.

PD-1 is a receptor on immune cells and its ligands, PDL1 and PDL2, are on a variety of cells, including antigen-presenting cells and tumor cells. Immune cell activity is reduced when PD1 binds to its ligand. The upregulation of PDL1 expression is seen in some tumor cells, which inhibits T-cell activation and increases cancer cell survival. , Monoclonal antibodies against PD-1 or its ligands, PDL1 and PDL2, upregulate the immune response and can inhibit tumor cell proliferation. Current agents that target these pathways are shown in Table 24.1 .
| Drug Class | Name |
|---|---|
| CTLA-4 Blockade | Ipilimumab Tremelimumab |
| PD-1 Blockade | Nivolumab Pembrolizumab |
| PD-L1 Blockade | Atezolizumab Durvalumab Avelumab |
Specific Endocrinopathies Associated With Immune Checkpoint Inhibitor Therapy
HYPOPHYSITIS
Hypophysitis, inflammation of the pituitary gland or pituitary stalk, has increased in incidence after being recognized as a complication from immune checkpoint inhibitor (ICI) therapy. The reported incidence of hypophysitis from anti-CTLA4 therapy has varied greatly from 0.4% to 17%. Tremelimumab has a lower reported incidence compared with ipilimumab (0.4% to 2.6% vs. 0.7% to 18.1%). More recent studies with an increased recognition of hypophysitis as a potential complication described incidence rates from 7.4% to 16.3 % following ipilimumab. , , Hypophysitis induced by anti-PD1/PDL1 monotherapy is less common (estimated at 1% or less), although more cases may be described as these agents become more widely used and studied more extensively. ,
Hypophysitis related to anti-CTLA4 therapy is an urgent endocrine condition, and most frequently presents with nonspecific symptoms such as headache, fatigue, and weakness. , , Other, less common symptoms can be nausea, poor appetite, weight loss, vision or mental status changes, temperature intolerance, and arthralgias. , , It may also be more common in men. However, this has been suggested to be related to the increased prevalence of men with melanoma in the trials examined. Age may also be a risk factor for hypophysitis after ICI therapy.
Inflammation and subsequent damage to the pituitary gland can manifest in a variety of hormonal deficiencies, the most dangerous being ACTH and TSH deficiency, which leads to secondary adrenal insufficiency and secondary hypothyroidism, respectively. ACTH and TSH deficiency are the most common pituitary hormone deficiency reported after anti-CTLA4–associated hypophysitis (CTLA4-H). Diabetes insipidus or posterior pituitary compromise is rare in these cases. , , , Hypophysitis from ICI therapy can be associated with clinically significant morbidity thought to be largely related to secondary adrenal insufficiency, with an incidence reported at approximately 6% across studies. Adrenal insufficiency and subsequent adrenal crisis can be life-threatening if left untreated/unrecognized. Symptoms of adrenal insufficiency can rapidly improve after steroid replacement with or without thyroid hormone. , Fifty percent of subjects can have hyponatremia that improves after hormone replacement. , Hypogonadism from pituitary damage may also occur. Insulin growth factor-1 (IGF-1) levels can be low but are measured less frequently, as growth hormone therapy is contraindicated in active malignancy. , Both elevated and decreased prolactin levels have been described after hypophysitis related to ICI therapy. , , ,
The secondary adrenal insufficiency that results from CTLA4-H is rarely reversible. Long-term glucocorticoid replacement is typically required. , Recovery from secondary hypothyroidism has been reported from 6% to 64% , ; reports of resolution from secondary hypogonadism vary from 12% to 57%. , , Thyroid and gonadotropin function assessment in ill patients can be complex as thyroid and gonadal laboratory tests during sickness can be similar to results seen in pituitary insufficiency (i.e., sick euthyroid syndrome/sickness-induced hypogonadism). Subsequently, differentiating recovery after illness from true recovery from CTLA4-H–induced thyroid or gonadal hormone deficiency may be challenging. However, with respect to cortisol levels, these values typically rise during illness.
In CTLA-4H, pituitary magnetic resonance imaging (MRI) can show mild to moderate diffuse enlargement of the pituitary gland with homogenous or heterogeneous appearance after contrast in 75% to 100% of patients. , Thickening of the pituitary stalk can be seen. Optic nerve compression is uncommon. The time frame of the MRI with respect to the diagnosis of hypophysitis and the radiologist’s experience with this entity may result in a lower possibility of positive findings on MRI. Pituitary enlargement has been shown to precede the clinical diagnosis of CTLA4-H, with the median time to onset of pituitary enlargement appearing 1 week before biochemical evidence of hormone deficiency. , An enlarged pituitary has been shown to decrease in size over 4 to 12 weeks, and subsequent atrophy of the gland can be seen. , , , Headache and pituitary enlargement may occur less commonly in nivolumab/pemrolizumab patients with hypophysitis (23% in one study).
CTLA4-H has been related to the ICI dose received, although there have been conflicting studies regarding this finding. , , Symptom onset has ranged from 6 to 14 weeks after anti-CTLA4 therapy, often occurring after the third treatment. , As described in one study, hypophysitis from anti-PD1 therapy without CTLA-4 blockade may occur later after therapy (median: 25.8 weeks, interquartile range [IR]: 18.4–44.0) compared with ipilimumab alone (9.3, IR: 7.2–11.1) or patients treated with a combination of both of these agents (12.5, IR: 7.4–18.6).
High-dose steroids have been used to try to reduce pituitary inflammation from CTLA4-H in the hopes of also preserving or reversing pituitary damage; however, they do not appear to improve the course of hormonal recovery. , , In addition, there is a concern that the immunosuppressant effect of high-dose steroids could negatively affect the antitumor efficacy of immune checkpoint inhibition. Accordingly, high-dose steroids should be reserved for those with clinically significant illness, hyponatremia, severe headache, or marked pituitary enlargement that approaches the optic apparatus. ,
PRIMARY THYROID DYSFUNCTION
Primary thyroid dysfunction is related to a thyroid gland abnormality, in contrast to secondary hypothyroidism that is related to hypophysitis/pituitary dysfunction. Primary thyroid dysfunction after ICI therapy is usually from thyroiditis and may be seen as diffuse uptake on a positron emission tomography (PET) scan. Thyroiditis can present first as thyrotoxicosis due to thyroid hormone release from inflamed thyroid tissue, which can then result in hypothyroidism. Graves’ disease is less common in these patients. Anti-CTLA4 and anti-PD1/PDL1 therapy can result in primary thyroid dysfunction; however, it may be more common with PD1/PDL1 blockade. , Studies of primary hypothyroidism after ipilimumab reported rates of approximately 5% to 6% occurring from 5 months to 3 years after treatment. ,
In initial studies, the overall rate was approximately 5% to 8% for hypothyroidism and approximately 3% for hyperthyroidism after PD1 inhibition. In thyroiditis, thyrotoxicosis can precede hypothyroidism in the same patient, as described previously. Subsequent studies looking specifically for primary thyroid dysfunction note that the rates could be as high as 14% to 20% after PD1 inhibition, particularly after combination ICI therapy. , Hypothyroidism onset can be as early as 3 weeks after treatment and up to 10 months following therapy; however, the majority of cases occur within the first 1 to 3 months of ICI treatment. , , Up to 50% of the cases of thyrotoxicosis may be transient, with patients subsequently returning to a euthyroid state. If primary hypothyroidism develops following anti-PD1 therapy, it is often permanent. , , A previous history of hypothyroidism or a high titer of thyroid peroxidase antibodies at baseline may predict a higher risk of worsening or recurrent hypothyroidism after anti-PD1 treatment. An 8.6% of incidence of hypothyroidism was reported after a trial with atezolizumab, an anti-PDL1 therapy. Additional clinical studies will be required to investigate whether the endocrine adverse events of anti-PDL1 therapy will be comparable with those seen with other ICI treatments.
PRIMARY ADRENAL INSUFFICIENCY
Case reports of adrenalitis have been published, and overall, the incidence of primary adrenal insufficiency appears rare. Ipilimumab, pembrolizumab, and nivolumab have been reported to result in primary adrenal insufficiency. Antiadrenal antibodies have been reported in a couple of studies. , Adrenal inflammation has also been described, , as well as diffuse increased uptake of bilateral adrenals on PET scan. , Adrenal atrophy has also been also reported in a subject. The adrenal insufficiency described in these reports is consistent with ICI-induced autoimmune destruction of the adrenal glands. ,
INSULIN-DEPENDENT DIABETES
Insulin-dependent diabetes following anti-PD-1/PD-L1 therapy (ICI-DM) is uncommon (less than 1% incidence). , ICI-DM typically presents with marked hyperglycemia or diabetic ketoacidosis (DKA) with low C-peptide levels, and appears irreversible. Patients can present with DKA or marked hyperglycemia as early as 1 week and up to 12 months following therapy initiation, with a median of 8.5 weeks with median glucose of 530 mg/dL. Symptoms of DKA or hyperglycemia include polyuria, polydipsia, blurred vision, and malaise. Positive autoantibodies to diabetic autoantigens can be seen with ICI-DM, as well as upregulation of CD8+ T-cell activity. , Serum glucose is often measured during standard laboratory monitoring during ICI therapy, and practitioners should follow glucose patterns. Aggressive management of DKA and individualized insulin regimens are required to manage ICI-DM. There are rare cases of IDDM following anti-CTLA4 therapy in the literature.
Combination Therapy
Combining CTLA4 and PD1 blockade can result in an additive benefit with respect to cancer therapy. However, combination therapy can increase the rate of adverse events. Additive benefits and increased adverse events with combination therapy were reported after trials of nivolumab/ipilimumab combination versus monotherapy. Approximately 59% of the combination arm had grade III–IV toxicities, in comparison with the monotherapy arm (nivolumab or ipilimumab, 21% and 28%, respectively). With respect to endocrinopathies in the combination arm, the trial reported a 17% incidence of hypothyroidism (nivolumab only 11%, ipilimumab only 5%); 11% incidence of hyperthyroidism (nivolumab 4%, ipilimumab 1.0%), and 7% incidence hypophysitis (nivolumab 1%, ipilimumab 4%). Also, a retrospective study showed that the rate of melanoma patients presenting with any type of thyroid abnormalities may be as high as 50% after anti-CTLA4/PD1 combination therapy.
Endocrinopathies and Treatment Response
An association between clinical response and IRAEs from ICI therapy has been reported in trials. In particular, in patients with ipilimumab-related hypophysitis, a prolonged median survival time (19.4 months vs. 8.8 months in patients without hypophysitis) was reported. Extended survival has been reported with anti-PD1 therapy and the development of thyroid dysfunction; however, studies have been conflicting. , In a study of patients receiving pembrolizumab for non–small cell lung cancer, the median overall survival in those who had thyroid dysfunction was significantly longer than in those without (median = 40 vs, 14 months, P = 0.029), although a possible lead time bias could also occur when studying these outcomes, as only those patients benefiting from prolonged survival from ICI therapy may be followed long enough to record the development of adverse events.
Diagnosis and Management
HYPOPHYSITIS
Hypophysitis has mainly been described after anti-CTLA4 therapy, either alone or in combination with anti-PD1/PDL1 agents. It is rare with anti-PD1/PDL1 therapy alone (1% or less), as described previously. Routine screening during therapy with CTLA4 and/or PD1/PDL1 blockade has included baseline and follow-up thyroid function tests (TFTs) but not adrenal function testing. It is important to consider monitoring morning ACTH and cortisol levels during treatment with CTLA4 blockade given that adrenal insufficiency can be life-threatening ( Fig. 24.2 ). Patients who have symptoms suggestive of hypophysitis should have a prompt evaluation for hypopituitarism. Initial evaluation involves morning ACTH and cortisol levels (ideally at or before 8:00 a.m.) as well as TSH and FT4. If hypophysitis is suspected, pituitary imaging with MRI can be performed. A formal visual field examination may be needed for patients who note visual field changes or have evidence of optic chiasm compression on imaging.
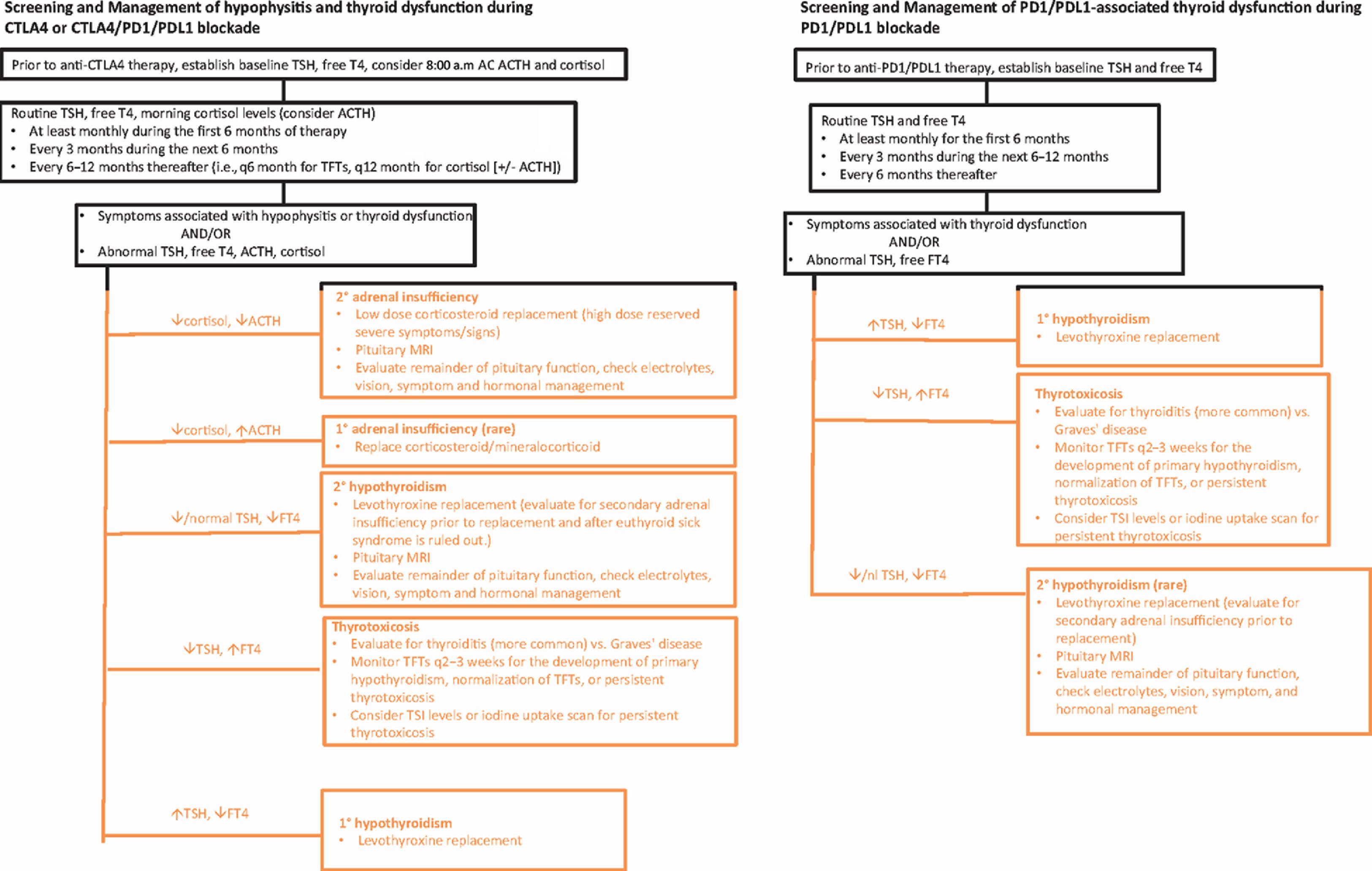
Laboratory evaluation can be considered monthly for the first 6 months on therapy given that hypophysitis tends to occur early in treatment after CTLA4 blockade ( Fig. 24.2 ). If laboratory tests are normal and a patient is asymptomatic, the interval could decrease to every 3 months for the following 6 months and further to every 6 to 12 months thereafter ( Fig. 24.2 ). If TFTs show low or low-normal TSH and low free T4, surveillance brain imaging shows an enlarged pituitary, or symptoms consistent with hypophysitis develop; morning (8:00 a.m.) paired ACTH and cortisol levels should be measured. Patients with secondary adrenal insufficiency from CTLA4-H often have a very low 8:00 a.m. cortisol (less than 3 mcg/dL) with a low ACTH (less than 5 pg/mL). Normal subjects have serum cortisol levels in the early morning (at or before 8:00 a.m.) in the 10 to 20 mcg/dL range. Random ACTH and cortisol levels can be measured if early morning laboratory testing is not possible or there is an urgent need at a clinical visit. These values can be frankly low for any time of day in patients with CTLA4-H. Clinicians should confirm that a patient has not been receiving exogenous steroids for a different indication, as this can influence ACTH and cortisol levels. Patients taking exogenous dexamethasone can have a low ACTH and cortisol level because of hypothalamic–pituitary-adrenal (HPA) axis suppression. However, a patient actively taking prednisone or hydrocortisone may have a low ACTH but a normal or high cortisol, as these medications can be picked up in the assay for cortisol. In contrast, dexamethasone is not detected in the cortisol assay. Long-term steroid use can result in suppression of the HPA axis. Corticosteroid administration for another IRAE can also mask the presentation of ICI-induced adrenal insufficiency or hypophysitis. ACTH-stimulation testing is not as useful in detecting early secondary adrenal insufficiency. This is because, initially, in pituitary injury, the adrenal glands may respond to ACTH stimulation because they have not atrophied from the long-term absence of pituitary ACTH stimulation. Gonadotropins, testosterone (in males), and estrogen (in premenopausal females) levels should be assessed in those diagnosed with hypophysitis. In those with secondary hypogonadism, prolactin levels can be measured, as hyperprolactinemia can result in hypogonadotropic hypogonadism. There is also a high incidence of hyponatremia in patients with CTLA4-H, which may be due to secondary adrenal insufficiency and/or secondary hypothyroidism. Diabetes insipidus is uncommon in CTLA4-H. , Growth hormone and IGF1 levels do not necessarily need to be assessed, as growth hormone replacement is contraindicated in patients with active cancer. If CTLA4 and PD1/PDL1 blockade is used, monitoring thyroid function and adrenal function following the recommendation algorithm for anti-CTLA4 monotherapy can be performed as in Fig. 24.2 .
In ICI-related hypophysitis, patients can have isolated hormonal deficiency or multiple pituitary hormone deficiencies. , Therapy goals include hormone replacement and symptom management. Acetaminophen, nonsteroidal antiinflammatories, or steroids can be used to manage headaches. To replace central/secondary adrenal insufficiency, hydrocortisone at approximately 10 mg/m 2 (i.e., 10 to 15 mg in the morning and 5 to 10 mg in the afternoon) or the equivalent dose of daily prednisone can be used. After hormonal replacement is initiated, ICI therapy typically can continue uninterrupted. High-dose steroids can be reserved for critical illness, significant hyponatremia, severe headache, visual disturbances or other neurologic symptoms, or marked pituitary enlargement that is close to the optic chiasm. ICI treatment may be withheld until the patient is stable on hormonal replacement in these more severe clinical situations. Glucocorticoid treatment can decrease pituitary enlargement to help ameliorate associated symptoms; however, high-dose steroids do not appear to reverse hypopituitarism. , Patients with adrenal insufficiency should receive education regarding the need to increase steroids during illness or surgical procedures and should have a medical alert bracelet. Evaluation for pituitary-adrenal axis recovery may be performed every 3 to 6 months for the first year and then every 6 to 12 months, although secondary adrenal insufficiency from CTLA4-H is typically permanent.
Levothyroxine should be used to treat central hypothyroidism after nonthyroidal illness is ruled out. Glucocorticoid replacement should be started before or simultaneously with thyroid hormone replacement in order to prevent precipitating adrenal crisis in those with both ACTH and TSH deficiency. Prolactin can be measured in patients with hypogonadism, as an elevated prolactin can cause secondary hypogonadism. Testosterone replacement may be used to treat male hypogonadism after ruling out hyperprolactinemia and eugonadal sick syndrome. Testosterone replacement therapy should track the Endocrine Society guidelines. If clinically indicated, estrogen replacement can be used in premenopausal women who have hypogonadotropic hypogonadism. Evaluation for pituitary-thyroid and pituitary-gonadal axis recovery may be performed in 3 to 6 months for the first year and subsequently every 6 to 12 months thereafter, as recovery of secondary hypothyroidism and secondary hypogonadism has been reported. Hyponatremia is typically transient and improves following hormonal replacement.
PRIMARY THYROID DYSFUNCTION
Hypothyroidism is the most common endocrine abnormality following either anti-CTLA4 or anti-PD1/PDL1 treatments. Baseline TSH and free T4 can be performed prior to either CTLA4 or PD1/PDL1 blockade and then at least monthly for the first 6 months. If the laboratory tests are normal and the patient is asymptomatic, TFTs can be checked quarterly for months 6 to 12 and about every 6 months thereafter. If a patient has any symptoms or signs of thyroid dysfunction in between visits, TFTs should be measured. Thyroid autoantibodies can be checked if a patient has primary hypothyroidism or thyrotoxicosis.
Clinical and biochemical abnormalities may improve in 2 to 4 weeks in those initially with thyrotoxicosis from thyroiditis, although prolonged thyrotoxicosis has been described. TFTs should be monitored at least every 2 to 3 weeks in those with thyrotoxicosis, as they can progress to hypothyroidism.
Graves’ hyperthyroidism and ophthalmopathy are rare after ICI therapy. , In those with prolonged thyrotoxicosis, goiter, or ophthalmopathy, TSIs, TRAb, or a thyroid uptake scan can be done to evaluate for Graves’ hyperthyroidism. One would expect a high iodine uptake in the thyroid gland in Graves’ hyperthyroidism. In thyroiditis, one characteristically sees a low iodine uptake. Radioactive iodine uptake scans in cancer patients can be inaccurate given their exposure to imaging using iodinated contrast, which lowers the thyroid’s iodine uptake.
Symptom management with a beta-blocker may be used for transient thyrotoxicosis from thyroiditis. Many patients with thyrotoxicosis from thyroiditis are relatively asymptomatic. Glucocorticoid treatment is rarely needed and may be considered in those with heart disease and/or severe symptoms who could warrant withholding of ICI treatment. An ATD, such as methimazole, can be used for Graves’ hyperthyroidism. However, these agents are ineffective for thyrotoxicosis from thyroiditis, which causes the majority of cases of ICI-thyrotoxicosis.
Levothyroxine should be started and titrated up every 4 to 6 weeks to normalize TFTs for primary hypothyroidism. Patients can be closely followed without thyroid hormone replacement if asymptomatic with an elevated TSH lower than 10 and normal free T4. In secondary hypothyroidism from pituitary dysfunction (low free T4 with a low or low-normal TSH), secondary adrenal insufficiency should be ruled out before giving thyroid hormone to avoid causing adrenal crisis. A low replacement dose of levothyroxine should be started and increased slowly in elderly patients or those with cardiac disease. Immunotherapy can be withheld in severe symptomatic cases of thyroid dysfunction, although this is uncommon.
PRIMARY ADRENAL INSUFFICIENCY
Monitoring guidelines have not been established for ICI-related primary adrenal insufficiency given its rare occurrence. If adrenal enlargement or atrophy is seen on routine scans, it is important to evaluate adrenal function by measuring ACTH and cortisol levels, as well as a cosyntropin stimulation test, to assess for primary adrenal insufficiency. Both corticosteroid and mineralocorticoid replacement are used to treat primary adrenal insufficiency.
INSULIN-DEPENDENT DIABETES
Even though IDDM is rare during ICI therapy, practitioners should be familiar with the signs and symptoms of DKA or hyperglycemia (polyuria, polydipsia, blurred vision, malaise), as missing this diagnosis can be life-threatening. Serum glucose is often measured during standard laboratory monitoring during ICI therapy, and practitioners should follow glucose patterns. Tests for autoantibodies (glutamic acid decarboxylase/GAD65 Abs, insulin Abs, islet cell Abs, zinc transporter 8/Zn-T8 Abs) and endogenous insulin secretion (C-peptide and insulin levels) can differentiate between insulin-dependent and non–insulin-dependent diabetes. Aggressive management of DKA and individualized insulin regimens are required to manage ICI-DM.
COMBINATION THERAPY
For patients on combined therapy with CTL4 and PD1/PDL1 blockade, clinicians can follow the suggested testing for monotherapy ( Fig. 24.2 ) and be mindful of the potential increased risk of endocrinopathies with combination ICI therapy.
Conclusions
Endocrinopathies induced by immune checkpoint blockade have been reported with increasing detail as indications for these agents expands across all fields of oncology. Even though the precise mechanisms are not known, increased activity of the immune system induced by ICI therapy can result in autoimmunity against normal tissue. Anti-CTLA4 treatment is associated with hypophysitis and primary thyroid dysfunction. PD1/PDL1 blockade is predominantly linked to primary thyroid dysfunction from thyroiditis. Combination CTLA4 and PD1 blockade therapies appear to confer an increased risk for both thyroid dysfunction and hypophysitis compared with monotherapy. Fulminant IDDM has been reported after PD1/PDL1 blockade, with only a few cases reports after CTLA4 blockade. Reports suggest that particular IRAEs can be associated with a superior response to cancer therapy. Additional studies are needed to fully describe this relationship. The overall frequency of endocrinopathies may not be fully appreciated given that earlier clinical trials may not have thoroughly monitored for endocrine adverse events. Clinicians need to be aware of these endocrinopathies as some can be life-threatening if undiagnosed. Fortunately, there are effective screening methods and hormonal replacement therapies for ICI-associated endocrinopathies. If detected and treated early, the morbidity associated with these disorders can be reduced, as well as limit the interruption of life-saving immunotherapy.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree