Chapter 10
Endocrinology
Alison Johnston and Jacqueline Lowdon
Diabetes Mellitus
Alison Johnston
Introduction
Type 1 diabetes is a disease of insulin deficiency due to autoimmune destruction of the pancreatic islet β cells which make the hormone insulin. It accounts for over 90% of diabetes in the under 25 years age group.
The incidence of type 1 diabetes in children and young people within Europe is increasing significantly. The Scottish incidence of type 1 diabetes by 10 year age bands per 100 000 population during 2007−2010 for children <10 years was 26, and for those aged 10−19 years was 43 [1]. The prevalence of diabetes in children and young people aged 0−17 years in England in 2007 was 207 per 100 000 [2]. It is predicted that between 2005 and 2020 prevalent cases younger than 15 years in Europe will rise by 70% [3].
Other types of diabetes do occur in young people: advances in molecular science mean that monogenetic forms of the condition are being identified; diabetes secondary to pancreatic disease is increasingly recognised (e.g. as in cystic fibrosis); and type 2 and other insulin resistance syndromes are on the increase [4].
Evidence based publications outline nutritional recommendations for people with diabetes; there are many of these including:
- the National Institute of Care Excellence’s document Type 1 Diabetes Diagnosis and Management of Type 1 Diabetes in Children and Young People [5]
- the Scottish Intercollegiate Guidelines Network, SIGN 116 Management of Diabetes, A National Clinical Guideline [6]
- the American Diabetes Association Position Statement Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications [7]
- Diabetes UK’s Evidence-based Nutrition Guidelines for the Prevention and Management of Diabetes [8]
- more specifically for children, the International Society of Paediatric and Adolescent Diabetes (ISPAD) clinical practice guidelines Nutritional management in children and adolescents with diabetes [9] (which have been adopted by Diabetes UK)
- Diabetes UK’s Evidence-based Nutrition Guidelines for the Prevention and Management of Diabetes [8]
There are no recent published dietary guidelines for children and young people with type 1 diabetes in the UK. Extensive literature searches carried out for the compilation of the guidelines highlight that the evidence for specific paediatric diabetes dietary management and education is limited and more scientific research is required. However, there is robust evidence for the nutritional requirements of children. Dietary recommendations for children with diabetes are based on the assumption that they have the same nutritional requirements as those who do not [10], and therefore healthy eating principles apply. There is also no debate about the vital role of nutritional management in diabetes care or that carbohydrate is the main dietetic consideration for glycaemic control. This chapter reflects current recommendations.
It is recommended that children and young people with type 1 diabetes attend a specialist children’s clinic and are cared for by an appropriately trained paediatric multidisciplinary diabetes team providing ongoing integrated education and support [11]. This team should include a paediatrician, diabetes nurse specialists and a paediatric dietitian; children and young people should have access to psychological services and social workers in addition to services offered in primary care. The team should work collaboratively with the child and their family to optimise diabetes control, whilst enabling them to manage their diabetes day to day and reduce the risk of complications. To be most successful, dietary guidance and education must be tailored to individual clinical, social, psychological, cultural and economic needs and dietitians should convert the scientific knowledge about food into practical, feasible advice.
The parents of a child who is diagnosed with a chronic disease (including a newly diagnosed diabetic child) are initially shocked and traumatised. Parents can also feel a sense of guilt: they may feel that their child has developed diabetes because they have permitted him or her to eat sweets excessively and should be reassured that this is not the case. Since the cause of diabetes is partly genetic, parents can feel guilty about passing on ‘bad genes’, a feeling exacerbated if there is a family history.
An effective, family-based dietary education programme which is going to result in the modification of a child’s eating habits can only begin properly when parents are allowed to grieve and come to terms with the diagnosis of diabetes in their child. It is vital to develop a rapport with the family so that a high quality of consistent dietetic care can be provided. Frequent and short teaching sessions are preferable, with the entire family if appropriate. Group sessions offering structured education provide an alternative method and further research is required to evaluate the most effective way for healthcare professionals to educate children [5]. Many families have fears about what their child will be allowed to eat and drink which must be allayed. Conducting the teaching sessions in the family’s own home has many advantages: less disruption, familiar surroundings, the child’s usual food and exercise pattern is maintained, first-hand knowledge of the domestic set-up and the session may become more learner centred. The disadvantages are that these sessions are costly in terms of travelling time and resources. A room organised and furnished as a teaching environment, away from the clinical setting, may prove to be a useful compromise.
The field of paediatric diabetes has changed markedly with the increasing incidence of type 1 and non-type 1, children being diagnosed at a younger age, the advent of analogue insulins, improved technology (such as insulin pumps and continuous glucose monitoring systems (p. 571) and intuitive blood glucose meters) and scrutiny of the effectiveness of methods of diabetes education. There has never been a time when the importance of strict glycaemic control has been so prominent and accentuated with the families. The dietitian has a vital role to play in the interdisciplinary team to optimise glycaemic control and quality of life.
Aims of dietetic management
The aims of dietetic management are the following.
- To meet the child’s nutritional requirements and to inculcate good dietary habits for good health. Children with diabetes have the same basic nutritional needs as their non-diabetic counterparts. It can be emphasised that the recommended eating plan is ‘healthy eating’ for the entire family. Eating a balanced breakfast, lunch, dinner and bedtime snack (with sensible mid-morning and mid-afternoon snacks if necessary or desired) will provide a healthy diet and offers a platform for regular blood glucose testing.
- To contribute towards optimising blood sugar levels and hence HbA1c (glycosylated haemoglobin) results <58 mmol/mol avoiding swings between hyperglycaemia and hypoglycaemia. Before basal bolus therapy and insulin pumps were the predominant models for insulin therapy, it was vital to distribute carbohydrate through the day to counter the inevitable periods of hyperinsulinaemia. While matching insulin to food is now much better, it remains important to consider how the insulin and food will interact. A preprandial blood glucose of 4−6 mmol/L is ideal; however, a target of obtaining 80% of the blood sugars in the range 4−8 mmol/L is probably more realistic. It is imperative to aim to maintain blood glucose concentrations close to the normal range to decrease the frequency and severity of long term microvascular and cardiovascular complications [12]. Recurrent episodes of hypoglycaemia are undesirable, particularly in young children where the developing brain may be particularly susceptible.
- To ensure dietary modifications allow normal growth and development. Dietary energy should be sufficient for growth, allow for variable exercise patterns and should not provoke obesity but maintain a healthy body mass index (BMI). Growth should be plotted at regular intervals using recommended height and weight centile charts. Growth velocity charts and BMI are useful for anticipating the onset of obesity or stunting. Growth can be a useful indicator of diabetes control, as poor physical development may be a consequence of inadequate diabetes management. Obesity is less of a problem in children with diabetes than in adults with diabetes, but if children do gain weight disproportionately to their height, suitable dietetic advice should be given at a very early stage.
- For the diet to contribute to minimising the development of diabetic complications such as cardiovascular and microvascular disease.
- To prevent and treat acute complications of diabetes, e.g. hypoglycaemia and hyperglycaemia and also to aid the management of diabetes during exercise and periods of illness.
- To provide a tailor-made eating plan which takes into consideration the psychological, cultural, social and emotional implications of food.
Dietary recommendations
Energy intake
The recommended distribution of total daily energy intake [13] is outlined in Table 10.1. It should be recognised that in children the energy distribution between carbohydrate, fat and protein will differ depending on age: breast fed infants will obtain approximately 55% energy from fat, 5% from protein and 40% from carbohydrate, whereas a 5-year-old may derive 35% energy from fat, 15% from protein and 50% from carbohydrate. Traditional dietary guidelines have emphasised only the carbohydrate component. Present day practice adopts a more holistic dietary approach. In addition the energy content of the diet should be tailored to the individual. This is of major importance in order to minimise the risk of chronic degenerative disease such as obesity and coronary heart disease.
Table 10.1 Recommended distribution of total daily energy intake
| Nutrient | Distribution |
| Total carbohydrate | 45%−60% of energy intake |
| Sucrose | Up to 10% of daily energy intake, provided it is eaten in the context of a healthy diet |
| Total fat | <35% of energy intake |
| Saturated and transunsaturated fat | <10% of energy intake |
| n-6 polyunsaturated fat | <10% of energy intake |
| Cis-mono-unsaturated fat | 10%−20% of energy intake |
| Protein* | 10%−15% |
* Recommendation from NICE guidelines [5].
Considerations about energy intake and body weight
The following need to be considered.
- At the time of diagnosis many children have suffered catabolic weight loss resulting in subsequent increased appetite and calorie intake [14]. This settles when their appropriate weight has been restored, usually within 1−4 weeks.
- Regular dietary review should encompass monitoring growth and revising advice to accommodate changes in age and lifestyle, nutritional requirement and insulin therapy. The review should include consistent advice about the prevention and treatment of hypoglycaemia to avoid over-treatment. The insulin regimen should be examined and if necessary adjusted to minimise hypoglycaemia and the need for large calorific snacks.
- Prevention of children and young people becoming overweight and obese is paramount and advice should be given about suitable portion sizes, eating healthy meals regularly, exercise and self-control.
- Withholding food in an effort to control blood glucose or make children eat when they are not hungry should be discouraged [15] as it may have a detrimental effect on growth and development. Furthermore it can make children resent having diabetes and they may perceive food differently because of these practices. Necessary adjustments should be made to insulin doses instead.
- Throughout puberty energy requirements increase significantly as do insulin requirements.
- In childhood the prevalence of obesity is increasing rapidly [16] due to excessive calorie intake and lack of physical activity. In addition for children with diabetes over-insulinisation, snacking and excess energy intake to avoid or treat hypoglycaemia can be contributing factors.
- Motivational interviewing can be used with older children when dealing with weight management.
- Maintaining a static weight in children who are overweight or obese whilst they are still growing taller is a success as they are in effect slimming down. This can be a more realistic and achievable target than actual weight loss. A growth centile chart should be used with the child and their family to show progress.
- Unexpected weight loss must be investigated and can be a sign of coeliac disease, insulin omission or an eating disorder.
Carbohydrate
Children and adolescents with diabetes are recommended to eat the same amount of carbohydrate as children eating a healthy diet who do not have the condition and carbohydrate remains a fundamental factor in optimising diabetes control. The type and amount have an effect on postprandial blood glucose levels.
The total amount of carbohydrate eaten in meals and snacks has a significantly greater effect on glycaemia than the source or type [16]. The recommended total energy intake from carbohydrate is 45%−60%.
Sources of carbohydrate from healthy foods should be encouraged such as wholegrain breads and cereals, peas, beans, lentils, fruit, vegetables and low fat dairy products. The Department of Health (now through the Food Standards Agency) recommends the consumption of five portions of fruit and vegetables per day [17].
Glycaemic index
The glycaemic index (GI) is a measure of the glycaemic response when consuming a particular food. It estimates how much each gram of available carbohydrate in a food consumed will raise the blood glucose level relative to the consumption of glucose or bread. Current validated methods use glucose as the reference food which has a GI of 100. Low GI (≤55) foods will release glucose slowly into the blood stream and will result in a lower postprandial glucose peak. Medium GI foods are classified as those with a GI of 56−69. High GI foods (≥70) cause a rapid rise in the blood glucose and can be useful to treat hypoglycaemia.
A number of factors affect the glycaemic response to food including the composition and amount of carbohydrate, the effect of cooking or processing, the ripeness of fruit and vegetables, physical entrapment of the starch molecules and other meal components such as fat and protein (fat and protein lower GI). The glycaemic response from a food differs from one person to another and in the same individual from day to day and is dependent on blood glucose levels and insulin resistance. The diet for a child with diabetes should not be based solely on the GI ranking of foods. GI can provide a useful guide to the effect a food will have on the glycaemic response but will not necessarily result in a nutritionally balanced diet. It should be borne in mind that the total amount of carbohydrate eaten is more significant, in terms of blood glucose response, than the type of carbohydrate eaten. Some foods considered to be relatively ‘unhealthy’ such as chocolate cake and ice cream have a low GI and other foods commonly included in a ‘healthy diet’ such as brown bread, baked potato and rice have a high GI.
Including low GI foods instead of higher GI foods in the diet may reduce postprandial peaks in glucose and HbA1c [18, 19]. Table 10.2 gives ideas of how to increase low GI foods in the diet.
Table 10.2 Increasing low glycaemic index foods in the diet
| Basmati rice instead of white or brown rice |
| Pasta and noodles instead of white rice or mashed potatoes |
| Sweet potato or new boiled potato instead of baked or mashed potato |
| Plenty of fruit and vegetables, lentils and beans |
| Granary or rye bread instead of white or wholemeal bread |
| Porridge, Special K and muesli instead of cornflakes and rice crispies |
| Fruit, low fat yoghurt and popcorn for snacks |
The GI does not take into account the amount of carbohydrate actually consumed. A related measure, the glycaemic load, takes this into consideration by multiplying the glycaemic index of the particular food by the carbohydrate content of the actual serving. However, the practicality of families calculating glycaemic load is questionable.
Sucrose
Avoidance of sugar and sugary foods was the main feature of dietary advice for many decades. More recently it has been shown that sucrose can provide up to 10% of the total daily energy intake without a detrimental effect on blood glucose control and conversely total abstinence of all sucrose containing foods may have a negative psychological impact and is not necessary. Sucrose has a similar glycaemic effect to isocaloric amounts of starch [20]. This information should be used in the context of a healthy diet and sugary drinks should be avoided (unless treating hypoglycaemia) as they may cause hyperglycaemia.
Fibre
Dietary fibre is an integral part of any healthy diet. The UK recommendations for fibre in childhood are imprecise and dated. The UK recommended dietary values for children state that fibre intake should be proportionately lower than that of adults, related to body size and that children <2 years of age should not be given fibre rich foods at the expense of energy rich foods [10]. There is no real evidence to substantiate the concern that a high fibre diet may lead to growth retardation and malabsorption of minerals; a higher fibre diet that reduces energy intake may be beneficial with the increasing incidence of obesity in children [21]. In the USA the recommendation is 2.8−3.4 g fibre/MJ (240 kcal) for children >1 year [22]. Soluble fibre in legumes, vegetables and fruit can aid reduction of lipid levels [23] and fruit pectin may improve protection against cardiovascular disease [24].
Many children eat a low fibre diet prior to diagnosis and gradual changes to increase fibre intake are necessary to minimise colic, flatulence and abdominal distension. Children can easily include a number of high fibre foods in their diet, e.g. wholemeal bread and pasta, high fibre breakfast cereals, baked beans and high fibre baked goods. A significant proportion of children do not achieve the recommended intake of five portions of fruit and vegetables per day but will eat at least one portion of fruit each day; many do not like vegetables, but will take them when included in soups and stews. Often raw vegetables will be taken in preference to those that are cooked. Many children will eat pulses in the form of baked beans, peas, sweetcorn, kidney beans or lentil soup.
Fat
Fat is necessary in children’s diets to provide adequate energy, fat soluble vitamins and essential fatty acids. The recommended amount of fat is 30%−35% of the total daily energy intake and studies have found that children with diabetes exceed the recommendations [25]. Detailed recommendations regarding the composition of the fat in the diet can be found in the ISPAD guidelines [9] and for many children the emphasis can be simply placed on reducing fat intake in general. The following advice can be given to reduce dietary fat:
- take grilled and oven baked foods in preference to fried foods
- cut off visible fat on meat
- eat fish and poultry instead of red meat
- cut down on the quantity of crisps eaten to 2−3 bags per week (often a compromise of a maximum of one bag per day has to be conceded); use reduced fat crisp varieties
- have reduced fat cheeses or varieties that are lower in fat (cottage cheese is not popular with children, so it is more realistic to limit the amount of high fat cheese and encourage lower fat varieties such as Edam or half fat hard cheese)
- use semi-skimmed milk over 2 years of age and semi-skimmed or skimmed milk in the over 5s, provided appetite is good and an adequate energy intake can be maintained
- choose fruit for snacks instead of biscuits or crisps
It is advisable for children to eat oily fish once or twice per week. Families should be informed of the importance of healthy eating in reducing the risk of cardiovascular disease, including advice about types and amounts of fat in the diet. People with diabetes are prone to dyslipidaemia so attention to dietary fat intake is as important as good metabolic control.
Protein
Children with diabetes should have protein intakes no higher than those taken by other children and should not exceed 15% of energy intake. In the diets of most children, protein provides 15% of dietary energy, although actual requirements are considerably lower than this [10].
Salt
The daily recommended maximum amount of salt children should eat [26] depends on age: 1−3 years, 2 g salt; 4−6 years, 3 g salt; 7−10 years, 5 g salt; 11 years and over, 6 g salt. This should be adhered to in order to reduce the risks of developing hypertension. An excess of salty foods should be avoided and only a little salt added in cooking if necessary. No salt should be added at the table.
Low sugar and diabetic products
Diet and low calorie drinks are valuable in the diet of a child with diabetes. Sugary squashes and fizzy drinks should be avoided completely (except when used to treat hypoglycaemia). Diet drinks sweetened with artificial sweeteners provide an alternative, whilst water is better still. Other low sugar and diet products marketed for the general population can also be useful, for instance reduced sugar jams, fruit canned in natural juice, low sugar desserts. Diabetic products, however, have no place in the diet for the child with diabetes. They are expensive and can be unpalatable and high in fat. In addition they may contain sorbitol or other sugar alcohols that have a laxative effect. The child should be encouraged to regard the diet as one of ‘sensible eating’ and not one which relies on the need to eat different or ‘special’ foods.
Sweeteners
Sweeteners are substances used to sweeten food. Sucrose (table sugar) can be eaten in moderation. Fructose has no advantage over sucrose in terms of taste as a sweetener, it contains as many calories and gives less satisfactory results in baking. Although it does not require insulin for its metabolism it has a glucose-sparing effect in the body and causes a rise in blood glucose if large quantities are taken.
Artificial and intense sweeteners
Polyols (sorbitol, xylitol, isomalt, maltitol, mannitol) do not increase blood glucose levels as much as sucrose or fructose. They are often added to foods such as diabetic chocolate and biscuits. Polyols have similar energy (or slightly lower) value to carbohydrate and are nutritive sweeteners. They are poorly absorbed and can cause osmotic diarrhoea, particularly in children, who have a lower body mass than that of the adult for whom the products are designed.
Non-nutritive sweeteners can be useful in drinks and desserts and to sprinkle on breakfast cereals. They are energy free, do not contribute to dental caries, are intensely sweet and do not affect blood glucose levels. In the UK five non-nutritive sweeteners are available: saccharin, aspartame, acesulfame K, cyclamate and sucralose. Many people find that saccharin has a bitter aftertaste. Aspartame, which many find more palatable, has a limited use because sweetening power is lost when it is subjected to prolonged heating. Acesulfame K is heat resistant and without aftertaste. Sucralose is 600 times as sweet as sucrose; it is stable under heat and can be used in cooking and baking and many find the taste palatable. These sweeteners are used in low sugar, diet or ‘light’ products to improve sweetness and taste.
Education
Dietary education should be a lifelong process commencing at the point of diagnosis. It is important to give the family ongoing practical dietary advice which is age related and the child should be involved, with age appropriate education, at every stage. The information should be delivered at a rate which considers the social, intellectual and cultural background of the child and builds on the family’s level of knowledge and understanding, taking into account their literacy and numeracy. Verbal instructions should be reinforced with appropriate written information and other resources should be used where possible, e.g. DVD, websites (see Further reading).
There is a lack of evidence to recommend a qualitative or quantitative approach as the most effective method of dietary education [6]. A qualitative approach teaches healthy eating along with the concept that carbohydrate is the component of food that increases the blood glucose level and the diet plan does not necessitate measuring or estimating carbohydrate amounts. Regular healthy eating and food with low glycaemic index is encouraged, with carbohydrate eaten at each meal and snack.
In practice it is agreed that carbohydrate counting is a necessary skill required to support intensive insulin therapy whether it be basal bolus or pump therapy. Carbohydrate counting can be challenging for those with limited mathematical ability but most families can do so to a greater or lesser extent.
There are two methods of managing the diabetes diet using carbohydrate counting: consistent carbohydrate intake and using insulin to carbohydrate ratios.
Consistent carbohydrate intake
The child or young person is recommended a set amount of carbohydrate to eat distributed at each meal and snack time following dietary review, agreement with the family and bearing in mind the action of insulin and the weight and height of the individual. The carbohydrate is counted using lists of portions of food that contain 10 g of carbohydrate and by calculating the carbohydrate content of food using the nutrition information on the product’s label. The daily amount of carbohydrate that an individual has been suggested should be regularly reviewed (ideally 4 monthly but not less than once per year) to ensure that it is apt. The child and their family learn to adjust insulin doses depending on the patterns of blood glucose readings to achieve target blood glucose levels.
Insulin to carbohydrate ratios
Insulin to carbohydrate ratio necessitates a more advanced level of teaching, understanding, monitoring and support to be successful. It involves calculating the ratio of rapid acting insulin to carbohydrate for each meal. The carbohydrate content of the meal is calculated and the appropriate amount of insulin administered. Adjustments can be made to the bolus dose of insulin allowing for exercise about to be undertaken and additional insulin given as a ‘correction dose’ if the blood glucose is above the target range. This liberates the child or young person from having to have consistent amounts of carbohydrate at each meal. However, reasonable regularity in mealtimes and eating routines are nevertheless important for optimal glycaemic control. The ratios must be calculated for each individual and for each mealtime and are determined by the child’s insulin requirement, duration of diabetes, sex, pubertal status and the time of day. The ratios can be calculated by carrying out ‘paired readings’, which is checking a preprandial blood glucose level and rechecking 90−120 minutes after the meal. If the preprandial and postprandial readings are the same (±2 mmol/L) then the dose of rapid acting insulin matches the amount of carbohydrate eaten.
Checklist
A checklist of dietary teaching that outlines topics to be covered with the family, which are dated and signed off on completion by the person conducting the education session, is worthwhile. This not only aids record keeping but also informs the entire diabetes team about the stage of dietary education a particular family has reached. A checklist for education is given in Table 10.3. An easily accessible, succinct record of any one to one or group teaching sessions that a child or young person and their family attended can be useful.
Table 10.3 Topics for education checklist
| Introduction to diabetes and food |
| Basic principles of the diet |
| Food groups and healthy eating |
| Foods which contain carbohydrate |
| How to count carbohydrate |
| How to work out the carbohydrate content of a portion of food using the nutrition information on a label |
| Signs, symptoms and treatment of hypoglycaemia |
| Eating out |
| Exercise |
| Dealing with diabetes at school |
| Illness |
| Using insulin to carbohydrate ratios |
Methods and tools
Education is essential to provide knowledge and skills to enable self-management and optimise glycaemic control and cardiovascular outcomes. Ongoing education is vital particularly because as a child grows he/she takes on more responsibility and becomes more independent. Proficiency is required in selecting the correct method and approach because if it is too difficult it may lead to failure or confusion and if too dogmatic it may cause the family to feel guilty and distressed [27, 28].
There is no consensus on the most appropriate methods or tools for education. There is recognition, however, that structured education based on sound educational principles and problem solving plays a key role in effective self-management and children and adolescents should have access to such programmes [6, 11, 29, 30].
Dose Adjustment for Normal Eating [31] (DAFNE) is a comprehensive structured education programme for adults, using basal bolus insulin and insulin to carbohydrate ratios. The principles of this programme have been modified to suit teenagers. Kids in Control of Food [32, 33] (KICk-OFF), based on DAFNE, has been subject to a national clinical trial and audited.
Hypoglycaemia
Hypoglycaemia is the most common acute complication of the treatment of diabetes in children. The recommended lower target for blood glucose levels in children and adults with insulin treated diabetes is 3.9 mmol/L [34] although there is no universally agreed definition. In clinical practice hypoglycaemia in children is generally defined as a blood glucose level <4 mmol/L as it provides families with a figure that is easy to remember; action should be taken in the interest of avoiding moderate or severe hypoglycaemia.
Causes of hypoglycaemia include:
- insufficient carbohydrate eaten, or being late for or missing a meal or snack. If on a basal bolus insulin regimen or an insulin pump the cause can be overestimation of carbohydrate intake or incorrect insulin to carbohydrate ratios
- exercise, without additional carbohydrate or reduction in insulin dose
- too much insulin or dose given at the wrong time
- food not absorbed, e.g. vomiting and diarrhoea
- alcohol
- too much insulin or dose given at the wrong time
Whatever the exact reason, hypoglycaemia in diabetes is always about relative hyperinsulinaemia (relative to the insulin level in the non-diabetic state).
The symptoms and signs of autonomic (andrenergic) activation (e.g. shaking, pounding heart, pallor, cold sweatiness) and/or neurological dysfunction (neuroglycopenia) (e.g. difficulty concentrating, disturbed vision and hearing, slurred speech, dizziness and in severe episodes loss of consciousness and seizure) can be experienced during hypoglycaemic episodes. Children can also have behavioural changes and mood swings including irritability, erratic behaviour, becoming upset and tearful. There are also non-specific symptoms such as hunger, headache, tiredness and nausea though these may be present when the blood glucose level is high. Children often find it difficult to describe their own symptoms, but might experience shaky or wobbly legs or a ‘funny’ feeling.
Hypoglycaemia is frequently categorised as mild, moderate or severe depending on symptoms and the ability of the individual to treat the ‘hypo’ themselves. Younger children will nearly always need to be treated by an adult and there are no important clinical reasons to distinguish between mild and moderate hypoglycaemia. If the hypo is moderate the child may be unaware of it, but appears to be confused and uncooperative. In severe hypoglycaemia the child is unconscious and may be fitting. Regular episodes of even mild hypoglycaemia indicate that the diet and insulin and perhaps exercise are out of balance and that a review of diabetes management is necessary. The possibility of surreptitious extra insulin administration should also be considered.
If hypoglycaemia is suspected the blood glucose should be tested to confirm it. Hypoglycaemia should be treated as soon as possible. Treatment depends on the severity of the hypo and the aim is to restore the blood glucose level to 5.6 mmol/L [35] (euglycaemia).
Extrapolation from adult studies shows that 0.3 g/kg of glucose in the form of glucose tablets will raise the blood glucose by 2.5−3.6 mol/L [36] in children. For example approximately 10 g of carbohydrate will be required to treat hypoglycaemia for a 30 kg child. The amount of carbohydrate required to restore euglycaemia will depend on the weight of the child, type of insulin therapy and time since its administration, recent activity and starting blood glucose. The type of carbohydrate is significant. Glucose is the preferred carbohydrate for the immediate treatment of hypoglycaemia as it does not require digestion or metabolism. Chocolate, milk and sweets containing fat should not be used to treat hypoglycaemia as the fat will cause the blood glucose to rise slowly. Twice the amount of carbohydrate from fructose (in the form of fruit juice) was needed compared with that from glucose tablets to raise blood glucose by similar amounts [37]. Sucrose and milk also require greater amounts to provide the same rise in blood glucose.
It is advised that a remedy for hypoglycaemia is available at all times, but for some children the temptation of carrying sweets is too great. It can be useful to view the hypo remedy as a ‘treatment’ and carry glucose tablets or a glucose drink.
Mild hypoglycaemia
In practical terms, mild hypoglycaemia should be treated:
- with 10 g of carbohydrate, e.g. glucose tablets such as 3 Dextro Energy tablets or 2.5 Gluco Tabs, or 60 mL of glucose drink such as Lucozade Original
- wait 10−15 minutes and then recheck the blood glucose level
- if it is >4 mmol/L the usual meal or snack should be eaten if it is due or 10 g of starchy carbohydrate, such as an apple, should be taken to maintain blood glucose levels until the next meal
- if the blood glucose is <4 mmol/L another 10 g of fast acting carbohydrate should be taken and the process repeated until the blood glucose is >4 mmol/L
- some diabetes services across the UK also include jelly beans, cola drinks and fruit juice in the list of suitable hypo remedies
Moderate hypoglycaemia
At the stage of moderate hypoglycaemia:
- the child will need help to take a sugary drink or food
- the treatment is the same as that for mild hypoglycaemia
- Glucogel, Dextrogel or Rapilose (prescribable tubes of rapidly absorbed glucose gel) can be most useful. Each tube contains 10 g of carbohydrate that can be squeezed into the mouth if the child is uncooperative
- this should be followed up with starchy carbohydrate once the child has recovered
- if there is any concern about ability to swallow then oral remedies should not be attempted because of the risk of choking
Severe hypoglycaemia
- Most centres advise that parents and carers keep an emergency supply of a GlucaGen HypoKit for severe hypoglycaemia.
- This is a glucagon injection to use at home if the child is unconscious or fitting or if the parents are unable to resolve hypoglycaemic symptoms by other means.
Recommending an eating plan
A dietary assessment is essential on diagnosis so that the child’s normal intake and meal pattern can be ascertained. The carbohydrate or energy allowance and distribution can then be tailored to the home situation. Providing the child is not overweight, the usual energy intake prior to the onset of diabetic symptoms can be used as a basis for deciding the diet.
Recommendations for particular insulin regimens
Recommendations for dietary management must take into consideration the child’s current insulin regimen.
Single dose
A single daily dose of intermediate or long acting analogue insulin injection regimen is outdated and seldom used. It necessitates a rigid set amount of carbohydrate to be eaten at regular intervals as three meals and three snacks throughout the day to reduce the risk of hypoglycaemia and hyperglycaemia.
Twice daily injections
Two injections each day of mixed rapid or short acting and intermediate acting insulins (one before breakfast and one before the evening meal) require regular fixed amounts of carbohydrate throughout the day to reduce the risk of hypoglycaemia and hyperglycaemia. This regimen is seldom used with children in the UK now as intensified insulin therapy is preferred, i.e. basal bolus regimens and pump therapy. Carbohydrate in excess of 50−60 g given at any one time may produce an inappropriately raised postprandial blood sugar when using mixed insulins. A meal pattern of three meals and three snacks each day is appropriate for most children, although very young children and adolescents may need more snacks. Carbohydrate should be distributed throughout the day taking account of the peak periods of insulin action.
Split evening insulin
A split evening regimen involves having three injections per day: a mixed (rapid or short and intermediate acting) insulin before breakfast; a rapid or short acting insulin with the evening meal; and an intermediate or long acting dose given either with the evening meal or before bed. The advice for twice daily insulin applies during the day but this system allows more flexibility with the amount of carbohydrate eaten at the evening mealtime. The amount of rapid or short acting insulin can be proportionally adjusted to suit the quantity of carbohydrate eaten. The advantage of such a regimen is that there is no need for a lunchtime injection at school, but good glucose control is unlikely to be achieved without adherence to regular and consistent amounts of carbohydrate day to day.
Basal bolus
Basal bolus therapy uses four or more injections per day. The basal or background insulin is given as intermediate or long acting insulin once or twice daily and boluses of rapid or short acting insulin are given before meals or snacks containing more than 10−20 g of carbohydrate. Some centres advocate giving boluses even with very small snacks, although many consider it impractical to expect a child to inject for all meals and snacks and therefore 20 g is often used as a limit above which a bolus should be given.
It is preferable to inject bolus doses prior to a meal to optimise glycaemic control by reducing the postprandial rise in glucose and lessening the possibility of an injection being forgotten. In the case of toddlers or young children, where it is difficult to assess how much they will eat, a compromise is to give a postprandial injection. Apart from using a pump, this method of giving insulin offers the most flexible way to eat as there is no need to adhere to rigid meal and snack times as rapid or short acting insulin is injected with food. The amounts of carbohydrate at meals can be varied and appropriate adjustments made to bolus doses using insulin to carbohydrate ratios. Another benefit of using rapid acting insulin prior to meals is that due to its short duration of action snacks between meals are not always necessary. This is useful for those trying to lose weight or for those who dislike eating snacks. Bedtime snacks, however, are often necessary to reduce the risk of nocturnal hypoglycaemia.
Paediatric diabetes services throughout the UK employ a variety of approaches to establish the insulin to carbohydrate ratio (ICR) for meals and managing snacks. ICR must be calculated for the individual child and for each meal. One method is to initially suggest fixed amounts of carbohydrate for the three meals in the day based on diet history or recall. Snacks of 10−20 g are suggested and not normally covered by bolus insulin. Basal insulin may be given once or twice daily and these doses are titrated to those required by the child. Some centres work out the ICR by examining the amount of insulin given with the meal and the blood glucose result of the test done at the following meal (or bedtime snack in the case of the dinner ICR). In others the family is asked to do an ‘8-point profile’. This profile is blood glucose testing before breakfast, lunch, evening meal and bedtime snack, 90 or 120 minutes post main meals and at 3 am. The pre meal blood glucose tests will indicate the appropriateness of the basal insulin and the post meal tests the effectiveness of the rapid acting insulin. The insulin dose is deemed correct for the amount of carbohydrate if the blood glucose reading pre meal is the same as that checked 90 or 120 minutes after the meal (±2 mmol/L). Once the rapid acting doses are adjusted to match the carbohydrate ICR can be established. For example if 4 units of insulin is required for a 40 g carbohydrate breakfast the ICR is 1u:10 g; if 3 units of insulin are required for a 60 g carbohydrate lunch the ICR is 0.5u:10 g.
Another way of stating the same ratio is in terms of the number of grams of carbohydrate which will match one unit of insulin. For example, an ICR of 0.5u:10 g can be expressed as 20 g:1u. Many insulin pumps and some blood glucose meters have in-built ‘bolus calculators’ to assist with the calculations and these normally use this grams per unit system.
Once the ICRs have been established the child can eat to appetite (within the context of a healthy diet) at mealtimes and inject with the suitable amount of rapid acting insulin. If snacks containing more than 10−20 g are desired a bolus of rapid acting insulin can be taken. Many teenagers will want to eat a bedtime snack that is more than 20 g of carbohydrate.
Frequently, different ICRs are required at the three mealtimes and normally breakfast requires more insulin per 10 g of carbohydrate compared with other times in the day because of insulin resistance from hormones such as cortisol and growth hormone. It is necessary to regularly check, by blood glucose testing and using the 8-point profile, that the ICR is adequate as children’s insulin requirements change as time goes on.
Insulin pumps
Insulin pumps, or continuous subcutaneous insulin infusion, offer the most freedom. The pump delivers a fixed or variable basal dose continuously throughout the day and bolus doses are administered each time carbohydrate is eaten. As no injection is required, it is advised to give a bolus with every snack, even very small ones.
As with basal bolus therapy the ability to count carbohydrate is paramount when using a pump. Accurate carbohydrate counting and ensuring that the individual’s ICR is correct for each time block in the day will contribute towards an optimal HbA1c result. Pumps also incorporate sophisticated calculators that assist in, for example, avoiding ‘stacking’ of insulin doses on top of each other.
Most pumps now have the ability to deliver the bolus in various ways. A straightforward single dose may be given but it is also possible to deliver the bolus over a prolonged period of time (sometimes known as a ‘square wave’) or have part of the bolus delivered immediately and the remainder over a period of time (sometimes known as a ‘dual wave’). This allows the bolus to be delivered to match the postprandial glucose excursion of the meal (dependent on the glycaemic index or fat content of the meal). A square wave is useful if the meal is going to be eaten over a prolonged period of time such as at a family celebration. If a child has an irregular eating pattern and it cannot be guaranteed that they will finish their meal the square wave will deliver the bolus over a period of time, perhaps an hour, and will be cancelled if necessary if the food has not all been eaten. It is also possible to deliver several small bolus doses if they eat little and often. This makes a pump ideal for infants and toddlers. A dual wave is effective for high fat foods that are carbohydrate dense such as pizza and creamy pasta dishes [38–40].
Studies in healthy subjects [41] and in those with diabetes revealed that food containing mainly carbohydrates causes a rapid and short glucose rise, whereas protein and fat containing meals result in a milder but prolonged increase in blood glucose levels. There is evidence emerging that meal related insulin dosing based on fat and protein counting, in addition to carbohydrate counting, reduces postprandial glucose levels [42]. Some centres across Europe are using carbohydrate plus fat and protein counting with normal, dual and square wave boluses and where prolonged boluses are utilised the duration is dependent on the amount of fat and protein in the meal. The more protein and fat the longer the duration of the bolus. The fat and protein counting algorithm used by Pańkowska et al. [43] has been adopted by some centres. Further studies are required to investigate translating findings from pump therapy to multiple injection therapy. Questions remain about how this can be implemented in practice.
The families have to experiment with different foods and bolus methods using blood glucose monitoring as their guide to get the best use of their pump’s features.
Exercise
Insulin, carbohydrate and exercise are the three fundamental influencing factors in blood glucose control. The many benefits of exercise, including weight control and reduced cardiovascular risk, far outweigh the potential risks of hypoglycaemia and hyperglycaemia associated with exercise. Families need dietary education about exercise management to contribute towards optimising control, providing adequate nutrition and, for older and competitive youngsters, optimising performance.
Exercise has the effect of lowering blood glucose levels by increasing the non-insulin dependent uptake of glucose by cells and increasing insulin sensitivity. During exercise, in type 1 diabetes, the pancreas does not control insulin levels and there may be impaired glucose counter regulation; as a consequence, hypoglycaemia frequently occurs during exercise or afterwards.
Children can have variable blood glucose outcomes as a result of additional activity. Levels can be maintained if the insulin doses are suitably adjusted and the intake of carbohydrate is sufficient. Hypoglycaemia can result due to excessive insulin, prolonged exercise without consuming additional carbohydrate, or moderate intensity aerobic exercise. Hyperglycaemia can be a consequence of a lack of insulin before and throughout the activity, the emotional reaction to competing or excitement causing an adrenalin surge, eating or drinking too much carbohydrate, an increase in adrenalin response due to brief, sporadic bouts of intense anaerobic exercise or after exercise when glucose production exceeds utilisation. Table 10.4 shows the factors associated with the effect of exercise on blood glucose levels.
Table 10.4 Factors associated with the effect of exercise on blood glucose levels
| Duration and intensity of the exercise |
| Type of activity (aerobic or anaerobic) |
| Metabolic control (there is a probability of ketosis developing if blood glucose levels are high and insulin levels are insufficient) |
| Type and timing of insulin doses |
| Type and timing of carbohydrate consumed |
| Absorption of insulin |
| Competitive nature of the sport (adrenalin will increase the blood glucose level) |
| Timing of the activity |
It can be easier to manage diabetes using a pump or a basal bolus regimen with children and young people who are extremely active and are involved in competitive sports.
Practical advice is key and families must learn to manage exercise by experimenting, regularly checking blood glucose levels and building on their experiences. A detailed log of carbohydrate intake, insulin doses and intensity and duration of activity can aid the family and the diabetes team in observing cause and effect of the various factors on the blood glucose. Accordingly changes can be instigated to improve the management of blood glucose control and exercise. Children should not exercise if the blood glucose is >14 mmol/L and they have ketonuria or ketonaemia. Extra insulin should be taken and the situation rectified before exercise is undertaken.
Dietary advice for exercise:
- Most periods of exercise or activity lasting more than 30 minutes will necessitate some adjustment to carbohydrate intake and or insulin.
- The type of activity, the weight of the child and the level of circulating insulin will determine how much carbohydrate will be necessary to avoid hypoglycaemia.
- Additional carbohydrate may be required before, during and after exercise; eating carbohydrate afterwards helps to replace hepatic and muscle glycogen stores.
- For younger children their energy expenditure is so variable from day to day and hour to hour that it is more practical, in most instances, to cover additional activity with extra carbohydrate. Often exercise can be easily dealt with by giving a snack of 10−20 g of carbohydrate as fruit, dried fruit, fruit juice, a cereal bar or bread. This can also be a time to allow a ‘fun size’ bar of chocolate; the fat slows down the absorption of the sugar and makes it more suited for longer lasting activities such as swimming or long walks.
- An isotonic drink containing 6% simple sugar such as glucose, fructose or sucrose is ideal if additional carbohydrate is required for short duration exercise. This is preferable to more concentrated juices and carbonated sugary drinks that may cause stomach ache and delay gastric absorption [44]. Many swimmers choose to drink this as the bottle can be left at the edge of the pool and sips easily taken throughout the session.
- Adolescents may require up to 1.0−1.5 g carbohydrate/kg/hour for moderate to intense exercise [45].
- Older children involved in strenuous exercise over a long period should have a balanced meal with protein, fat and carbohydrate 3−4 hours beforehand to maximise the endogenous energy stores and permit time for digestion. Up to an hour before the activity a carbohydrate drink (1−2 g carbohydrate/kg) may be taken to increase energy stores and provide fluid for hydration; this will boost glycogen stores [44].
- For some older children additional pre-exercise carbohydrate may necessitate bolus insulin if the exercise is high intensity or strenuous or anaerobic [46]. Hyperglycaemia and poor performance can be caused by insufficient insulin.
- Fluid intake should be adequate as dehydration can impair performance.
- Following moderate or intense exercise carbohydrate stores must be replenished to lower the risk of hypoglycaemia, as insulin sensitivity remains raised for several hours.
- High intensity, brief, anaerobic exercise such as sprinting may not need carbohydrate beforehand but may result in a fall in blood glucose afterwards. In this case it is preferable to prevent hypoglycaemia by taking additional carbohydrate after the sport.
- Activities of a longer duration such as swimming, cycling, jogging, football may require additional carbohydrate before, during and afterwards.
- To minimise post exercise hypoglycaemia low GI foods, a reduction in basal insulin and a lower than usual bolus dose with the post exercise meal can be helpful.
- A remedy to treat hypoglycaemia must be carried at all times or be close by whilst exercising and it is advisable to also have some form of ‘diabetes identification’.
- Adults supervising children with diabetes including teachers, coaches, activity holiday staff should all be aware of the possibility of hypoglycaemia and know how to treat it.
- Hypoglycaemia can occur several hours after exercise (up to 24 hours) due to increased insulin sensitivity. Additional carbohydrate and/or a reduction in insulin may be required to prevent this. The risk of post exercise nocturnal hypoglycaemia is high and extra carbohydrate eaten at bedtime can reduce this threat.
- To minimise post exercise hypoglycaemia low GI foods, a reduction in basal insulin and a lower than usual bolus dose with the post exercise meal can be helpful.
The ‘Exercise in children and adolescents with diabetes’ [47] ISPAD guideline provides evidence based recommendations for people with diabetes.
Illness
Children often do not wish to eat during periods of illness and infection. Blood glucose levels are likely to be high at these times, so it is important that insulin therapy is continued. It is easier to manage diabetes during illness when a child is on a basal bolus regimen or on an insulin pump as additional insulin can be readily and safely given (with proper instruction and education) to prevent ketones developing or rid the body of them. For those on insulin twice or three times per day, a change in the insulin regimen to several doses of rapid acting insulin may be advised. Carbohydrate intake must not be cut to control the blood glucose level and is essential to prevent the body using fat reserves as a source of fuel with consequent ketone production. Blood glucose should be controlled by the insulin therapy using correction doses as recommended by the diabetes team.
If the usual diet is refused it is not essential to completely replace all the carbohydrate. A realistic aim is to give 70%−80% of the normal intake. Small frequent doses of rapidly absorbed carbohydrate, preferably as a liquid, are often best tolerated. Water and/or low calorie/sugar free drinks should be encouraged to prevent dehydration. Diarrhoea and vomiting illnesses can cause blood glucose to fall. At this time insulin should never be stopped (to prevent ketosis) but doses may be reduced and carbohydrate given as sugary drinks such as Lucozade or flat cola drinks.
Parents should always contact their diabetes team if concerned about their child’s condition. During recovery the child should return gradually to foods normally eaten.
Diet throughout childhood
Regular and continued dietetic input is essential in order that the dietary advice is appropriate for the child’s continuing and changing needs and lifestyle. Throughout childhood the dietitian should be involved in the diabetes education of the child and his/her carers, including nursery and school staff.
Babies
Adequate nutrition to promote growth is of major importance during the early months of life. Infants with diabetes should have their carbohydrate intake based on requirement for milk feeds, which are the principal source of nutrition. If at the time of diagnosis the infant is breast fed the mother should be encouraged to continue. However, many mothers are anxious about hypoglycaemia if they are uncertain of the amount being consumed at each feed and will need reassurance. Whether breast or bottle fed, a baby’s fluid requirement is 150−200 mL/kg/day; 150 mL breast or infant formula contains approximately 10 g carbohydrate. Providing growth and development is normal, the daily carbohydrate intake can be based on the baby’s usual feeding pattern with the insulin dose adjusted accordingly. As with all infants, breast milk or infant formula should be the milk of choice until the child is 12 months old.
An insulin pump is the delivery method of choice for babies and many children under 5 as their varying insulin requirements can be met more easily and unpredictable eating patterns dealt with. Basal bolus insulin therapy is an alternative. Breast fed infants can be given a bolus of insulin before or after each feed. For bottle fed babies the amount of carbohydrate in the formula feed can be calculated, insulin to carbohydrate ratios can be established and the appropriate amount of insulin matching the feed delivered by a pump or insulin pen. In some instances diluted insulin is necessary if the total daily requirement is small.
Weaning
Weaning advice for the general population applies to infants with diabetes. Most term infants need no nutrition other than breast milk or formula milk until 6 months (26 weeks) of age. Weaning can start around this time. Non-carbohydrate foods may be used initially (e.g. 1−2 teaspoons puréed vegetables) as these will not alter the carbohydrate intake. This allows the baby to become accustomed to the different taste and texture of solids without any anxiety being generated by food refusal. Once the baby has become familiar with spoon feeding, carbohydrate containing foods such as baby rice and rusk may be introduced. Water and dilute low sugar squashes can be given as an additional drink. Weaning is an anxious time for any parent and this anxiety is heightened if a dietary modification has to be observed. Tension about food must be relieved as a baby may refuse solids completely if the mother is fussing or worrying.
The Department of Health recommendations for vitamin supplements for infants and young children [48] also apply to the child with diabetes. The first year of life is the period of most rapid growth. Dietary intake is constantly changing due to the child’s progression through normal feeding and developmental milestones. It is essential that the dietitian is in frequent contact with the family to offer advice.
Hypoglycaemia is a real fear for parents and can be hard to recognise in babies. It may only be noted when parents are routinely checking blood sugars. Advice should be given on how to recognise and treat symptoms. Extra milk, with or without additional sugar, or glucose polymer, Ribena or sweetened fruit juice may be used. Usually 10 g carbohydrate given as 150 mL baby milk or 75 mL baby milk plus one teaspoon (5 g) of glucose polymer is sufficient to treat hypoglycaemia. Nocturnal hypoglycaemia is a concern; milk and a cereal can be given before the baby settles for the night if (s)he is no longer having night feeds and blood glucose levels are dropping overnight.
Toddlers
After 1 year of age the child can drink cow’s milk and from 2 years semi-skimmed milk can be introduced as long as the child is taking a nutritionally adequate diet. Fully skimmed milk or 1% fat milk, however, contains too little fat (and hence energy) for the under 5s and is not usually recommended. The introduction of skimmed milk over the age of 5 is a useful way to reduce the overall fat content of the diet. There is little fibre in the diets of children in their first year of life, but this can gradually increase. Some forms of fibre such as Weetabix, raisins, bananas and baked beans are popular with children; vegetables and wholemeal bread need to be encouraged.
Small children do not understand the importance of their diet, so parents and health professionals must be flexible and compromise as much as is practical. Erratic eating patterns are common in children between 2 and 4 years of age. Rigid mealtimes and snacks often do not work and well presented finger foods can be offered throughout the day. Most families manage to cope with the ‘food refusal syndrome’ without being manipulated, but the toddler with diabetes poses a real problem; with hypoglycaemia always a possibility, food refusal can become a powerful weapon. Parents are torn between maintaining good glycaemic control with the accompanying risk of hypoglycaemia, and allowing the blood sugars to be a little raised. Advice should be given not to force feed, nor to offer numerous alternatives, and to avoid fuss around mealtimes. The child’s falling blood glucose causing hunger and a desire to eat can be relied upon. In a small minority of cases toddlers’ food refusal can be a persistent and unremitting problem causing parents enormous worry about hypos. If the child is not on an insulin pump, one practical solution is to allow the child to eat meals with no fuss and when the meal is over to give an appropriate dose of rapid acting insulin analogue to cover the amount of food consumed.
Some young children with diabetes complain of incessant hunger. This may occur if control is poor and glucose is being lost in the urine or if the child is using food as a means to seek attention. Measurements of height, weight and dietary intake should be done regularly to reassure parents that adequate nutrition is being maintained.
Toddlerhood can prove to be a challenging stage when it is often difficult to achieve good glycaemic control and the families need plenty of support from their diabetes team.
Schoolchildren
While at school the teacher becomes one of the child’s main carers and must know about the condition and in particular how to recognise and treat hypoglycaemia. There are governmental acts across the UK that place requirements on education authorities to support children in schools and nurseries with conditions such as diabetes [49–51]. Concern has been raised amongst a number of schools about managing diabetes since the advent of pump therapy and increasing numbers of children on basal bolus therapy requiring ‘medical’ intervention at school. However, liaison with diabetes teams and information shared with the school resolves these worries and the child’s diabetes can be cared for capably at school. Diabetes UK provides information about managing diabetes at school and on school trips. Moreover many diabetes teams have their own literature and information available on websites. This information should be given to each child’s teacher. The dietitian and/or the diabetes nurse specialist may offer to visit the school to provide advice.
Children with diabetes are advised to carry a hypo remedy (glucose tablets) at all times. The teacher should be equipped with glucose tablets and/or a glucose drink in case of hypoglycaemia. Teachers of physical education should be aware of the need for extra carbohydrate before exercise and to check that children are carrying extra carbohydrate during periods of prolonged exercise.
School lunches can, but should not, present a problem; it is not always possible for children to go home and they may prefer to have school lunch with their friends. Older children with a good grasp of their diet can choose their own meal and the cafeteria-style canteen allows a flexible choice of food. The organisation and provision of school meals can vary from area to area; it is not desirable for the child to have a ‘special diet’ away from friends or at a different time from them. A normal main course should be suitable, with the dessert or pudding being replaced by fresh fruit or diet yoghurt. Discussion with the school cooks as well as teachers should help smooth problems. Advice can be given to parents about suitable packed lunches. Some local authorities provide information about the carbohydrate content of portions of foods available at school lunches; all authorities should be encouraged to do so.
Children frequently eat sweets in the school playground and it is tempting for a child with diabetes to eat sweets also. Other less sweet snacks such as fruit, bread, low sugar cereal bars, raisins, yoghurt and sugar free chewing gum should be suggested as alternatives. The best attitude is to use positive reinforcement when the child is doing well and to recommend that the best time for sweets is prior to exercise or after a main meal. If habitual bingeing is causing a problem the services of a child psychologist may be needed; bingeing suggests underlying stress.
Adolescents
As in other age groups, height and weight monitoring is important for early recognition of weight loss or disproportionate weight gain. Poor growth may be a sign of delayed puberty, coeliac or thyroid disease, omission of insulin, disordered eating or poor diabetes control. Excessive weight gain requires a careful examination of insulin dosage, dietary intake and physical activity. Occasionally, simply cutting out crisps and fried foods and exercising can achieve weight control. The amount of carbohydrate in the diet should only be reduced after very careful examination of the diet as a whole, as this may cause an increase in consumption of fat and protein. In order to get the message across, it is useful to point out the energy content of some of the carbohydrate portions, e.g. one packet of crisps provides 10 g carbohydrate and 150 kcal (630 kJ); one apple also contains 10 g carbohydrate but only 50 kcal (210 kJ).
Teenagers soon become aware of the fact that poor control can result in weight loss. A number will omit insulin as a method of weight control. The benefits of weight loss achieved by healthy lifestyle (eating and exercise) should be reinforced and the risks associated with poor control, acute and chronic, outlined. Some teenagers, both male and female, can have a distorted view of their weight. Eating disorders can present just as in the non-diabetic population with bingeing, anorexia nervosa and bulimia. Diabetes is a condition which makes weight control possible without the avoidance of food. Diabetes may increase the risks of eating disorders in children and young people, particularly in young women, as there is a greater focus on food, on weight (weighing people at each clinic visit, rapid weight gain with insulin) and a fear of hypoglycaemia often coupled with low self-esteem. Young people suffering from an eating disorder need careful monitoring and intervention by the diabetes team and the psychiatrist/psychologist.
Adolescence is a time of physical, social and emotional change. Teenagers with diabetes may not want to be different from their peers, worrying about gaining acceptance whilst fighting for independence with parents. Education about diet and re-education is important at this stage as an assumption can be made that because someone has had diabetes for a long time they know all about it when in fact it was the parents who learned about the condition. Support from the diabetes team whilst young people learn to manage their own diabetes is crucial during this period.
Snacking and eating out in the evening is common and advice about how to manage this is needed. Advice about alcohol and its hypoglycaemic effect will be necessary for teenagers, as it is known that many youngsters imbibe before they reach the legal age to do so. Alcohol is dangerous in excess as it suppresses gluconeogenesis and may induce prolonged hypoglycaemia in young people with diabetes. Carbohydrate should be eaten before, during and after alcohol intake especially if exercise is performed during or after drinking, e.g. dancing. Awareness of the alcohol content of drinks particularly targeted at young people should be raised.
Arrangements for transition of care to adult services varies widely across the UK but there should always be good communication between the paediatric and adult dietitians to ensure continuity of care.
Parties and eating out
Parties are highlights in a child’s life and advice is required to ensure that children with diabetes can enjoy the party as much as everyone else. They can eat most of the party fare and it is important to ensure that sufficient carbohydrate is eaten to compensate for extra activity and excitement. The host can help by providing low calorie drinks for everyone. Parties can involve sleepovers and advice should be discussed about food intake, timing of insulin doses and managing additional activity.
Many fast food eating places and restaurants publish the carbohydrate content of their foods in leaflets and on websites. Children should be educated about their diet as soon as possible so that they are able to manage it independently, with support from parents as necessary. All children are different, but most children from the age of about 8 should be actively encouraged to have a full understanding of their diet. Parents may need encouragement gradually to hand over responsibility of treatment to the child.
Travel
Travel should pose no problems with a little extra planning. Carbohydrate foods, e.g. sandwiches, fruit or biscuits, should be carried to cover inevitable delays, as should a hypo remedy. More food is required on activity holidays and usually a reduction in the insulin dose (of around 20%) too to prevent hypoglycaemia. When holidaying abroad advice should be given on practical issues related to long distance travel, e.g. when best to eat and inject insulin whilst travelling across time zones, how to avoid food poisoning and as always the children should wear some form of identification, e.g. SOS Talisman or Medicalert.
Non type 1 diabetes
Paediatric diabetes dietitians are more frequently being faced with the dietary management of children with non type 1 diabetes as the incidence is increasing.
Type 2 diabetes
There is little evidence regarding the dietary recommendations for children with type 2 diabetes and therefore advice suggested is derived from treatment of overweight and obese children, children with type 1 diabetes and adults with type 2 diabetes. The aims of dietary management are
- to achieve optimal blood glucose levels and normal HbA1c
- to prevent further weight gain in those with BMI on the 85th−95th percentile
- weight loss for those with BMI >95th percentile with normal linear growth [52, 53]
- to deal with comorbidities such as hypertension and dyslipidaemia
Most children with type 2 diabetes are overweight and dietary intervention aims to achieve weight loss or keep weight static with normal linear growth. Often the whole family is overweight and to teach and counsel the entire family about calorie reduction and increasing energy expenditure may be beneficial, rather than concentrating solely on the child or young person. There is some evidence that substitution of low GI foods for high GI foods may help with weight, lipid levels and control of appetite [54, 55].
Dietary management should incorporate:
- Cutting out sugary soft or fizzy drinks and fruit juices completely. This single dietary change can make the most significant change and result in weight loss. These drinks can be replaced with water, diet drinks and sugar free beverages.
- Healthy eating advice for the whole family with guidance about regular eating and avoiding the use of sweet foods as a reward or token of affection. Meals should ideally be eaten as part of a family routine, eaten in one place (preferably at a table) and with no distraction such as watching television, reading or playing. High fat and high calorie foods should be limited in the home. Education and instruction about reading nutrition information on food labels should be given to help the family when making food choices whilst shopping.
- Advice about exercising portion control within the family and that food should not be eaten out of a tin or packet but should be served on a plate or bowl.
- Keeping food diaries as this can raise awareness or food issues and help monitor progress.
- Positive reinforcement should be given for success, no matter how small, such as no or minimal weight gain, eating less of a high calorie food; it is seldom useful to criticise failure.
A number of children with type 2 diabetes are on oral medication and or insulin therapy. Those on insulin should have detailed education about carbohydrate counting and hypoglycaemia.
Diabetes secondary to pancreatic disease
Cystic fibrosis related diabetes (p. 239) is due to insulin deficiency but insulin resistance during acute illness and medication may also contribute to impaired glucose tolerance and diabetes. Blood glucose levels should be controlled, as high levels will promote catabolism and affect response to infection. It has been documented that early initiation of insulin therapy may have value for growth, weight and pulmonary function [56, 57]. Insulin doses and types may need to be adjusted when nutritional supplements, overnight or continuous enteral feeding are being used and during times of acute infection. Dose adjustment should take priority over dietary restriction.
Dietary management includes:
- ensuring sufficient calorie and intake to meet the individual’s requirements
- avoiding sugary drinks
- advice about the effect of carbohydrate on blood glucose levels and matching insulin dose with ingested carbohydrate.
Regular healthy eating advice may be sufficient; however, carbohydrate counting and use of insulin to carbohydrate ratios may also be necessary.
Diabetes secondary to medication
Some drugs, such as high dose steroids, can cause transient hyperglycaemia for the duration of the prescription and others, such as tacrolimus and cyclosporin, may cause islet cell destruction and the diabetes is permanent (p. 663). In oncology protocols some chemotherapy drugs induce diabetes which coincides with the cyclical nature of the chemotherapy. Following transplantation such as renal transplantation (p. 271), diabetes most commonly occurs with the use of high dose steroids and tacrolimus. Dietary management depends on how the diabetes is medically managed. In some instances instruction about avoiding sugary drinks and foods and healthy eating is sufficient. Other children are commenced on daily long acting analogue insulin and require additional information about eating regularly, having a bedtime snack and hypoglycaemia. Basal bolus or insulin pump therapy can also be initiated and the advice is similar to that given to those with type 1 diabetes.
Coeliac disease
Coeliac disease occurs in 10% of children with diabetes [58]. It is associated with poor growth, delayed puberty, nutritional deficiencies and hypoglycaemia; however, the presence of coeliac disease can often be asymptomatic. A gluten free diet must be incorporated into the diabetes eating plan as it is the only treatment for coeliac disease. It is understandable that many of the children who develop coeliac disease do not adhere to the gluten free diet [59] but should be encouraged to do so. Commonly these children are upset about having two conditions which require dietary modification and they may benefit from referral to the psychologist attached to the diabetes team to discuss the dual diagnosis. Advice should be given about the gluten free diet, gluten free products and the nutritional adequacy of the diet especially for iron, calcium, vitamin D, fibre and vitamin B intake (p. 117). Children with diabetes and coeliac disease should have frequent reviews by a paediatric dietitian with experience in both fields. Vitamin D and iron status should be checked regularly.
Dietary review
There should be an initial dietary teaching package for newly diagnosed children and young people with diabetes and they should have the opportunity to see a dietitian at each outpatient appointment; they should have access by telephone for advice at all times. As a minimum there should be an annual dietetic review. More frequent consultations are required in situations such as a change of insulin regimen, an excessive weight gain or loss, or where there is a need for further dietary education, e.g. poor knowledge or advice about diet and exercise, activity holidays, dyslipidaemia, comorbidities such as coeliac disease.
Summary
The dietetic care of children and young people is multifaceted and must be ongoing. Youngsters with diabetes should have an optimal quality of life in which a suitably tailored eating plan is an essential part. The dietitian has a unique role to play in the multidisciplinary team in the management of childhood diabetes and the education of the child and the family. They can offer support and reassurance along with dietetic expertise. Nutritional assessment and support must be an integral part of initial and continuing education programmes. Diabetes control and compliance can be less than ideal but this does not detract from the importance of balanced nutrition in this serious chronic disease.
Further reading and information
- Food for Life, book of dietary information by Alison Johnston and Anne Morrice. Available from GGC Children’s Diabetes Service, Royal Hospital for Sick Children, Glasgow G3 8SJ, and on website http://www.childdiabetes-scotland.org/ggc
- Supporting Children and Young People with Diabetes in Education by Alison Johnston and Nicola Thomson. Available from GGC Children’s Diabetes Service, Royal Hospital for Sick Children, Glasgow G3 8SJ, and on website http://www.childdiabetes-scotland.org/ggc
- Patient Held Record, a diabetes manual by Ian Craigie and Fiona Lamb. Available from GGC Children’s Diabetes Service, Royal Hospital for Sick Children, Glasgow G3 8SJ, and on website http://www.childdiabetes-scotland.org/ggc
- ISPAD Clinical Practice Consensus Guidelines 2009 Compendium available at www.ispad.org
- Ives Diabetes Exercise and Sports Association (www.diabetes-exercise.org), an international organisation that provides guidance and networking between beginners, health professionals and experienced athletes with diabetes
- www.runsweet.com, a website which shares knowledge on how to manage diabetes with different sports and exercises
Useful addresses
- Diabetes UK
- Central Office, Macleod House, 10 Parkway, London NW1 7AA
- Tel: 020 7424 1000
- www.diabetes.org.uk
Congenital Hyperinsulinism
Jacqueline Lowdon
Introduction
Congenital hyperinsulinism (CHI) is the most common cause of severe, persistent hypoglycaemia in newborn babies and children [60]. It is a clinically heterogeneous disorder characterised by severe recurrent hypoglycaemia associated with elevated serum insulin, C-peptide and pro insulin levels that are inappropriately high for the concentration of blood glucose.
Clinical presentation
CHI can be associated with several well defined clinical conditions such as uncontrolled maternal diabetes, perinatal asphyxia and Beckwith−Wiedemann syndrome, which all manifest with transient hyperinsulinism [61–63]. However, the focus here is on persistent hypoglycaemia so these conditions will not be covered.
Most infants with CHI present during the first few days of life, others during the first year. Very rarely, some older children may present with hypoglycaemic symptoms. The first clinical indications of CHI can include non-specific features of hypoglycaemia such as floppiness, jitteriness, poor feeding and lethargy [64]. If left untreated, CHI can lead to permanent neurological damage, or coma and death, secondary to severe hypoglycaemia [64–66]. Some infants with hyperinsulinism might present with macrosomia [67].
Hyperinsulinism in infancy can be clinically very heterogeneous, ranging from a severe life threatening disease to very mild clinical symptoms which may be difficult to recognise. Some of the most difficult to manage are those infants of normal or low birth weight, including preterm infants [64].
Incidence
Estimated incidence is 1 in 50 000 live births in western Europe [68, 69] with the highest incidence rate of 1 in 2500 births found in countries with high rates of consanguinity [70].
Early referral to a specialist centre
Due to the challenges of managing infants with CHI, early transfer to a specialist centre is recommended [71]. There are two centres in the UK: the Northern Congenital Hyperinsulinism (NORCHI) service which is a joint service between the Royal Manchester Children’s Hospital and the Alder Hey Children’s Hospital, Liverpool; and Great Ormond Street Hospital for Children, London. Paediatric dietitians may have initial contact with a new CHI infant and thereafter have shared care with the specialist centre dietitians.
Figure 10.1 outlines the admission management pathway for a new CHI patient to the Royal Manchester Children’s Hospital.
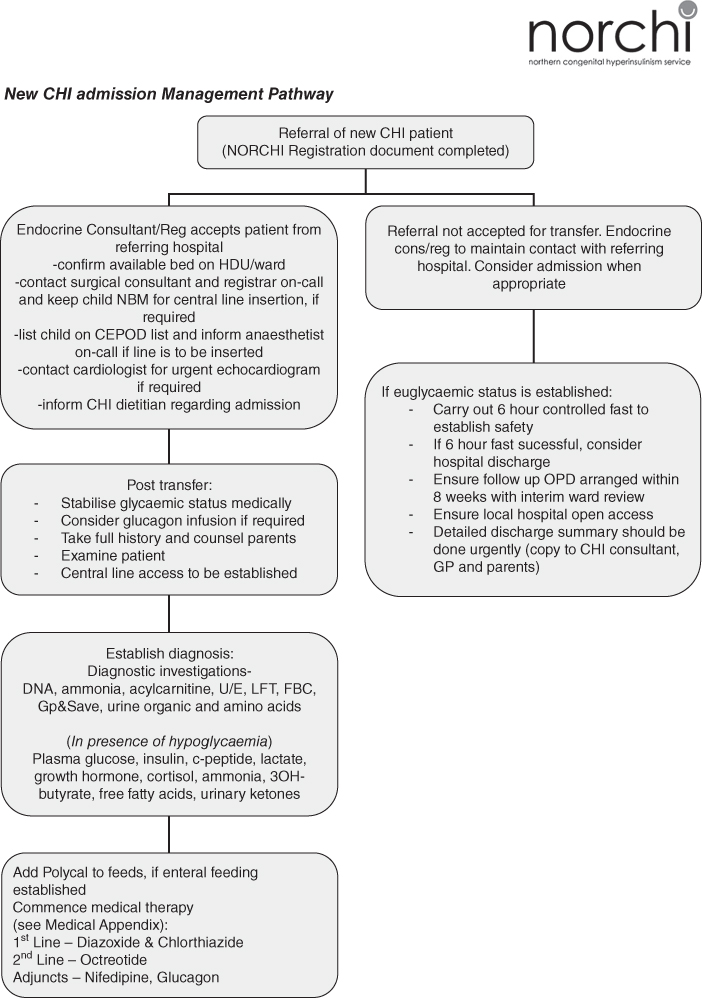
Figure 10.1 New CHI patient admission management pathway. Reprinted with permission of Royal Manchester Children’s Hospital.
Establishing diagnosis
There is a well established consensus for the primary and secondary diagnostic indicators for hyperinsulinism (HI) [71] shown in Table 10.5. Blood taken at the time of hypoglycaemia must be assayed for the substances listed in Table 10.6.
Table 10.5 Criteria for diagnostic indicators for hyperinsulinism
|
Table 10.6 Intermediary metabolites and hormones measured at the point of hypoglycaemia
| Blood | Urine |
| Glucose | Ketone bodies |
| Lactate/pyruvate | Reducing substances |
| Ketone bodies | Organic acids |
| Free fatty acids | |
| Amino acids | |
| Ammonia | |
| Total/free carnitine | |
| Acyl-carnitine profile | |
| Insulin/C-peptide | |
| Cortisol |
Genetics and pathogenesis of CHI subtypes
The clinical heterogeneity of CHI includes a variable age of onset, degree of severity and sensitivity to medical treatment. Two distinct histopathological types have been described [65, 72, 73]. Most exhibit diffuse involvement of the beta cells throughout the pancreas (diffuse HI), while some cases have focal adenomatous hyperplasia (focal HI). With focal HI a distinct area of the pancreas is involved, while the rest of the pancreas is histologically and functionally normal.
Genetic heterogeneity has also been described with over 40 different mutations [74] in seven different genes being detected in patients with CHI so far. Both autosomal recessive and dominant inheritance has been described [75] with the majority being recessively inherited [74].
In 30%−50% of patients there are abnormalities in either genes controlling intracellular metabolic pathways or membrane cation transport [76]. Most cases of CHI are caused by genetic mutations that result in an abnormal formation or function of KATP channels [77]. Consequently the beta cells secrete insulin even when blood glucose levels are low. The KATP channels are controlled by two genes: the sulphonylurea receptor gene (SUR1 now known as ABCC8) [78] and the inward-rectifying potassium channel gene (KiR6.2 now known as KCNJ11) [79].
Most cases of CHI due to KATP channel defects are recessively inherited. KATP-HI can result in diffuse or focal disease, depending on which parent the mutation is inherited from. Heterozygous mutation on the paternal allele can cause focal disease. Within the focal lesion there is selective deletion of maternal copy.
Other CHI cases are caused by mutations in the main enzymes involved in glucose metabolism [77], the beta cell enzymes glucokinase (GK-HI) and glutamate dehydrogenase (GDH-HI). Genetic mutations in genes coding for the GDH and GK enzymes are dominantly inherited and result in diffuse disease [77].
Although a number of gene mutations have been associated with CHI, the commonest mutations causing CHI are in the KATP channel genes. Rapid KATP channel gene mutation testing has significantly improved the investigation and management of CHI [80].
Testing for mutations in ABCC8 and KCNJ11 and identification of parental origin is useful in assessing the likelihood of focal and diffuse HI early in the treatment. Further, 18F-DOPA positron emission tomography (PET) CT scan imaging of the pancreas in select cases, e.g. those with paternal heterozygous mutations or no identifiable mutation, is critical to the diagnosis of focal HI [81–84]. The purpose of this scan is to determine whether there is a focal lesion within the pancreas that would be responsive to surgical resection. All children who have a CHI genotype consistent with possible focal disease should be considered for this scan. The scan should also be given to infants where no genetic mutations have been found but who remain on treatment for their CHI [85]. In the UK the scan is only available at the two specialist centres.
Management of CHI
Management aims are:
- prevention of hypoglycaemic brain damage, allowing for normal psychomotor development
- establishment of normal feeding regimen appropriate for the age of the infant/child – volume, frequency, type
- ensuring normal and safe tolerance to fasting that is appropriate for age, without developing hypoglycaemia
- maintenance of family unit and quality of life [71]
- optimal growth
Effective management is essential for a good outcome. Neuroglycopenia occurs with associated hypoketosis when blood glucose levels are below the normal range of 3.5–6.0 mmol/L. A high degree of psychomotor or developmental delay has been reported in up to 44% of cases, with epilepsy in up to 25% of cases [86] including infantile spasms occurring in a number of individuals [80]. Most recent data suggest persisting learning difficulties in 29% of patients [87].
Medical treatment
Initial stabilisation
The objectives of medical management are [71]
- stabilisation of blood glucose concentrations above 3.5 mmol/L
- establishment of a feeding regimen that is acceptable to the family without compromising the safety of the child, achieving normal blood glucose concentrations during a reasonable length of fast.
If high glucose infusion rates are required (>20 mg/kg/minute) [71], the insertion of a central venous catheter may be required for intravenous (IV) dextrose and/or IV glucagon; this may need to be considered even if the requirement for glucose is less.
A normal hepatic glucose production rate in a full term newborn infant is 4−6 mg/kg/minute [88]. However, in CHI the requirement is much higher, sometimes as high as 25 mg/kg/minute. It is important to estimate the glucose requirement to maintain euglycaemia and increased glucose requirement can be an important diagnostic step. The glucose requirement will continue to be a fundamental feature requiring careful and regular assessment in order to prevent severe hypoglycaemia, symptomatic and asymptomatic.
To calculate glucose requirement for IV fluids:
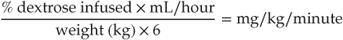
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree