
Endocrine Treatment for Metastatic Breast Cancer
 ABSTRACT
ABSTRACT
A tenth of women diagnosed with breast cancer have metastatic disease at the time of diagnosis, whereas some with locoregional disease at diagnosis progress despite treatment. The goal of metastatic breast cancer (MBC) treatment is palliation, but in a small subset of patients, it could be curative. Systemic therapy is the mainstay of treatment in MBC. The choice of therapy depends on the biology (receptor status), anatomy, rate of progression, patient preference, and the patient’s performance status. Endocrine treatment is the standard initial treatment for nonvisceral, non-rapidly progressing hormone receptor-positive MBC. Our chapter discusses the various forms of endocrine treatments and presents an approach to treating MBC.
Keywords: metastatic, breast cancer, endocrine
 INTRODUCTION
INTRODUCTION
In the United States, approximately 190,000 women are diagnosed with and 40,000 women die of breast cancer annually. About 1 in 10 women are diagnosed with metastatic breast cancer (MBC) at the time of initial diagnosis (1, and some with locoregional disease progress despite treatment.
The goal of MBC treatment is palliation, but in a small subset of patients, it could be curative. The most common sites of metastases are bone, lungs, and liver. When patients recur, it is generally recommended that the diagnosis of MBC be confirmed with a biopsy. If tissue is available from the recurrence, it is important to repeat the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) status.
Treatment can be broadly divided into local and systemic approaches. Local treatment consists of radiation, surgery, or a combination of the two, with symptom control as the primary aim. The role of local treatment in oligometastatic disease remains controversial.
Systemic treatment is the mainstay of therapy in the metastatic setting and is based on the biology (hormone receptor and HER2 status), anatomy (visceral vs nonvisceral), and rate of growth (rapid vs indolent) of the tumor, and the patient’s performance status.
For hormone receptor-positive disease, initial treatment with endocrine therapy is considered standard. For patients who are also HER2 positive, trastuzumab (Herceptin) or lapatinib (Tykerb) may be included as part of the treatment. The role of HER2-targeted therapy in patients who are both hormone receptor positive and HER2 positive is detailed elsewhere.
Chemotherapy is the treatment of choice in hormone refractory MBC or patients with rapidly progressing visceral disease. This review will focus on the role of endocrine treatment in hormone receptor-positive and HER2-negative MBC.
 ROLE OF ENDOCRINE TREATMENT IN MBC
ROLE OF ENDOCRINE TREATMENT IN MBC
Over a century ago, George Thomas Beatson described about breast cancer regression following oophorectomy (2). This led researchers to investigate the role of estrogen in the pathogenesis of breast cancer. Surgical approaches such as transsphenoidal hypophysectomy and adreno- oophorectomy were studied as treatment for advanced breast cancer (3,4). Subsequently, medical treatment for hormone receptor-positive breast cancer became the standard of care. Several modalities have now been developed to antagonize the estrogen-dependent activation of breast cancer cells.
Endocrine treatment for MBC is different for premenopausal and postmenopausal patients. Tamoxifen is commonly used in both premenopausal and postmenopausal patients. Aromatase inhibitors (AIs) and ER down regulators are utilized for postmenopausal patients. In premenopausal patients, ovarian ablation or suppression should be considered and include oophorectomy, ovarian radiation, or use of luteinizing hormone (LH)-releasing hormone analogues.
 PREMENOPAUSAL MBC
PREMENOPAUSAL MBC
Tamoxifen
Tamoxifen, formally called ICI-46,474, was initially developed as a contraceptive in 1962 by Arthur Walpole’s team and is the first selective estrogen receptor modulator (SERM). It did not thrive as a contraceptive and was found to be effective in advanced breast cancer (5,6). In 1983, the first clinical trial was published showing a survival benefit in patients treated with tamoxifen post-mastectomy in early breast cancer. These findings confirmed the role of endocrine therapy in breast cancer (7).
Tamoxifen works by inhibiting breast cancer cell growth via competitive antagonism of estrogen at its receptor site. It also has tissue-specific partial agonist activity which has beneficial effects, such as prevention of bone demineralization, but is also associated with an increased risk of thromboembolic events, uterine cancer, and uterine sarcoma (8).
Tamoxifen is beneficial in both premenopausal and postmenopausal hormone receptor-positive MBC. Tamoxifen is standard first-line treatment for premenopausal women who have never received tamoxifen or who relapse 12 months or longer after completion of adjuvant tamoxifen. It can also be used for postmenopausal women who have relapsed during or within 12 months of completing AI therapy. Studies suggest a 50% to 60% response rate (9–16). Duration of response has been reported between 12 and 18 months, but can be longer (9,17–19).
The recommended dose of tamoxifen is 20 mg orally daily. Higher doses are not more effective and can cause increased toxicity (17,19–21). The drug is metabolized to its active form endoxifen by the cytochrome P450 2D6 (CYP2D6) (22). Slow metabolizing and extensive metabolizing cytochrome genotypes have been identified. Cytochrome P450 2D6 genotyping is commercially available (e.g., AmpliChip). Recent data suggest that the efficacy of tamoxifen is decreased
when given in combination with selective serotonin reuptake inhibitors (SSRIs) secondary to CYP2D6 inhibition (23). Reported CYP2D6 inhibition potency with SSRIs from highest to lowest is as follows: paroxetine, fluoxetine, sertraline, citalopram, and venlafaxine. Other drugs that cause strong CYP2D6 inhibition include quinidine, risperidone, and tenofovir. It is important to consider the variations of tamoxifen metabolism in each patient.
Tamoxifen side effects include hot flashes (33)%), amenorrhea (16%), altered menses (13%), hypertension (11%), peripheral edema (11%), bone pain (6%), and nausea (5%). More serious adverse effects are deep vein thrombosis (5%), pulmonary embolus (0.5%), cataracts (6%), angioedema (<1%), Steven–Johnson syndrome (<1%), stroke (<1%), endometrial hyperplasia/cancer, or sarcoma (<1%) (24).
Although uncommon, a transient flare reaction, characterized by an increase in bone pain and/or increase in the size or number of metastatic skin lesions, can occur in up to 15% of patients. Time of onset is reported between a few days to up to 3 weeks. Hypercalcemia is found in 5% of patients with a tamoxifen flare reaction. Treatment is symptomatic and includes pain control and correction of hypercalcemia. Symptoms typically resolve in 4 weeks (25). Tamoxifen can be continued if symptoms are mild, but should be held and cautiously reintroduced when severe reactions occur.
Tamoxifen Resistance
Resistance to tamoxifen can be primary (failure to respond) or secondary (progression in a previously responding patient). It has been reported that 15% of patients with primary resistance and one third of patients with secondary resistance to tamoxifen benefit from other endocrine therapies. This leads to the hypothesis that alternative signaling pathways are involved, and one such association is HER2-mediated growth factor signaling which is thought to promote ER-independent growth in breast cancer cells. It is also postulated that HER2 down regulates ER expression and promotes endocrine therapy resistance, although the clinical evidence is conflicting (26). Current guidelines from the American Society of Clinical Oncology recommends against the use of HER2 status to withhold or preferentially select one endocrine treatment over another (27).
A withdrawal response to the discontinuation of tamoxifen therapy, as with high-dose estrogen and other estrogen agonists, is observed in some patients. Disease stability for more than 6 months has been reported, although the response can be shorter (28).
Other SERMs
Of the newer SERMs (idoxifene, raloxifene, tore-mifene), only toremifene (Farenston) is approved for the treatment of advanced breast cancer. Toremifene is less estrogenic as compared with tamoxifen and theoretically less toxic. However, clinical trials and meta-analysis in MBC have found comparable activity and toxicity profiles. Toremifene is cross-resistant with tamoxifen and therefore is ineffective as a second-line therapy after progression on tamoxifen (29–31).
 ESTROGEN DEPRIVATION OR OVARIAN ABLATION
ESTROGEN DEPRIVATION OR OVARIAN ABLATION
Oophorectomy
For any premenopausal patient with hormone receptor-positive MBC, estrogen deprivation or ovarian ablation should be considered. It can be permanent in the form of ovarian ablation by oophorectomy or temporary by the use of a GnRH agonist. Oophorectomy is a time-honored method of estrogen deprivation. It results in objective responses in about one third of unselected premenopausal patients with MBC. It has largely been replaced by tamoxifen as first-line therapy in premenopausal MBC because of similar efficacy and patient convenience. However, patients may benefit from oophorectomy after progression on tamoxifen (32,33). In addition, oophorectomy clearly expands the endocrine therapeutic options. Once patients become postmenopausal after oophorectomy, they become candidates for AIs.
GnRH Analogs
GnRH analogs can be used as an alternative to oophorectomy in premenopausal women with MBC. Goserelin and leuprolide are peptide analogs of natural GnRH but are far more potent than the natural hormone. GnRH is released in a pulsatile manner and acts on the pituitary gland to stimulate follicle stimulating hormone (FSH) and leutinizing hormone LH secretion. Although associated with an initial rise in FSH and LH secretion, GnRH analogs cause receptor down regulation through constant stimulation. This leads to profound suppression of the pituitary ovarian axis. Serum estrogen levels fall to menopausal levels (34,35). Efficacy of GnRH analogs is similar to oophorectomy or tamoxifen (36,37). Common side effects include hot flashes (75%) and tumor flare (15%) that is similar to estrogen therapy and tamoxifen.
 COMBINATION OF TAMOXIFEN WITH OVARIAN ABLATION
COMBINATION OF TAMOXIFEN WITH OVARIAN ABLATION
In premenopausal women with MBC, combination therapy with tamoxifen and GnRH analogue has resulted in a higher response rate (39%) vs 30%), significant decrease in hazard ratio (HR) of progression (0.7; P = .0003), and a small benefit in median survival compared with a GnRH analogue alone (38).
Additionally, Klijn et al. reported that the combination of buserelin with tamoxifen therapy resulted in better response rates, progression-free survival (PFS), and overall survival (OS) compared to LHRH alone (38). Combination therapy was not associated with increased side effects (39).
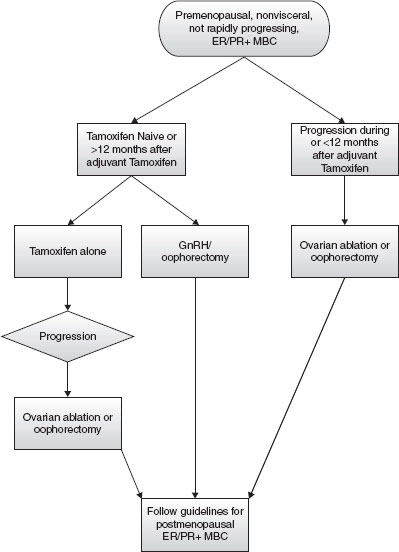
These studies support the use of combination therapy with ovarian suppression or ablation and tamoxifen as a first-line therapy in premenopausal women with MBC who are naive to tamoxifen or in those who relapse after 12 months of adjuvant tamoxifen therapy. However, given the invasive nature of oophorectomy and small benefit in OS, sequential therapy with oophorectomy or ovarian suppression can also be considered. A description of the different endocrine approaches and a treatment algorithm for premenopausal MBC are shown in Table 1 and Figure 1, respectively.
TABLE 1
Endocrine treatment for premenopausal ER+/PR+ metastatic breast cancer
Treatment | Indication | Response Rate |
Tamoxifen | First line previously untreated or >12 months from adjuvant treatment | 50–60% |
Oophorectomy | First line in combination with or 2nd line after progression on tamoxifen | Approximately 33% |
GnRH analogues | First line in combination with or second line after progression on tamoxifen | Same as oophorectomy |
Combination of tamoxifen and ovarian ablation/oophorectomy | First line previously untreated or>12 months from adjuvant treatment | 39% |
FIGURE 1
Endocrine treatment algorithm for premenopausal receptor-positive metastatic breast cancer patients. (Adapted from NCCN guidelines Breast Cancer v 2.2010).
Aromatase Inhibitors
As ovarian function declines after menopause, the majority of circulating estrogens are derived from peripheral conversion of adrenal androgens to estradiol and estrone (40–42). Aromatase enzyme mediates this conversion in adipose tissue, liver, and muscles. This forms the rationale for using AI therapy in treating postmeno -pausal MBC.
AIs decrease circulating estrogen levels and inhibit the growth of estrogen-dependent breast cancer cells. They can be classified as nonselective (first generation) and prevent the synthesis of all adrenal steroids, including estrogen or selective (second and third generation) based on increasing specificity for the aromatase site (Table 2).
Nonselective AIs
Aminoglutethimide
Aminoglutethimide is a first-generation nonselective steroidal AI. It was the first drug in this class to show efficacy in hormone-sensitive breast cancer. At lower doses, it causes inhibition of the aromatase enzyme resulting in antitumor activity (43–45). At higher doses, it blocks the conversion of cholesterol to pregnenolone by inhibiting the enzyme P450scc reducing synthesis of all hormonally active steroids. Aminoglutethimide is clinically used for adrenal suppression in patients with Cushing’s disease. It is also effective, although not as commonly used anymore, for the treatment of hormone-sensitive breast cancer. Side effects include skin rash, hepatotoxicity, and hypoadrenalism (decreased cortisol and aldosterone levels), which may be seen even at the lower doses (46). This has led to the development of safer, selective AIs.
Selective AIs
Selective AIs are specific to aromatase sites at regular doses and cause less systemic toxicities as compared with the nonselective AIs. They can further be divided into steroidal or nonsteroidal based on their molecular structure. Selective AIs are classified as second or third generation based on increasing specificity for the aromatase site.
Nonsteroidal Selective AIs
Fadrozole
Fadrozole is a second-generation (selective) nonsteroidal AI. It has been shown to be effective in patients with tamoxifen-resistant advanced breast cancer (47,48). When compared with tamoxifen as a first-line therapy in advanced breast cancer and untreated MBC, it was found to have similar efficacy (49). However, its clinical use has been superseded by third-generation nonsteroidal AIs because of proven superiority in randomized trials as well as increased specificity for aromatase site and a better toxicity profile.
Table 1
Overview of aromatase inhibitors
Generation | Nonsteroidal | Steroidal |
First (nonselective) | Aminoglutethimide | |
Second (selective) | Fadrozole | Formestane |
Third (super-selective) | Anastrozole | Exemestane |
Letrozole | Vorozole | |
Anastrozole is a third-generation nonsteroidal AI. It has been studied as a first-line therapy in post-menopausal women with MBC and in postmenopausal women with tamoxifen-resistant disease. Randomized trials as first-line therapy in post-menopausal MBC have found anastrozole to be at least as effective as tamoxifen with a better side effect profile, that is, fewer thromboembolic events and episodes of vaginal bleeding (11,12,50–54). When compared with megestrol acetate in post-menopausal women with tamoxifen refractory disease, anastrozole given at 1 mg daily produced higher objective response rate (CR + PR) of 10.3% versus 7.9% and significantly longer median survival of 27 versus 23 months (55).
Letrozole
Letrozole is another third-generation nonsteroidal AI. Its mechanism of action and clinical indications are similar to other third-generation selective AIs. Although letrozole causes more in vivo inhibition of aromatase and more suppression of estrone levels, clinical superiority is unclear (56,57). In an open randomized trial comparing letrozole 2.5 mg daily with anastrozole 1 mg daily in locally advanced or MBC, Rose et al. reported significantly higher objective response rate of 19% versus 12% with letrozole (58). However, no survival advantage or advantage in terms of time to progression was seen.
Mourisden et al. compared letrozole versus tamoxifen as first-line therapy in women with post-menopausal advanced breast cancer (15). Median time to progression was 9.4 months in the letrozole arm compared with 6.0 months in the tamoxifen arm (P < .0001). Overall objective response rate was 32% versus 21%, respectively (P = .0002). Median OS was slightly prolonged in the letrozole-treated arm compared with the tamoxifen-treated arm (34) vs 30 months), although not statistically significant.
In a randomized double-blinded trial comparing letrozole with megestrol acetate in tamoxifen-resistant advanced breast cancer, letrozole 2.5 mg daily produced significantly higher response rate of 24% versus 16% and better OS of 25.3 versus 21.5 months, but not statistically significant (59).
It has also been shown to be superior to aminoglutethimide in tamoxifen-resistant patients with advanced breast cancer (60). The side effect profi le is similar to other selective AIs.
Vorozole
Vorozole is also a third-generation nonsteroidal AI. It causes imidazole-based competitive inhibition of aromatase enzyme. A randomized phase III clinical trial comparing vorozole with megestrol acetate in postmenopausal advanced breast cancer showed no significant difference in median survival (61).
Steroidal Selective AIs
Formestane
Formestane and exemestane are steroidal AIs. They are androgenic steroids resistant to the action of aromatase and irreversibly bind to it, causing permanent inactivation (62–64). Formestane is a second-generation (selective) steroidal AI. It is unavailable in the United States. It is marketed in Europe as Lentaron by Novartis AG. In tamoxifen-resistant patients with advanced breast cancer, formestane at 250 mg given intramuscularly (IM) every 2 weeks has shown an objective response rate (CR + PR) between 23% and 39% (65–68). Phase III studies have shown similar efficacy to aminoglutethimide and megestrol acetate in tamoxifen-resistant patients (67). Formestane is as effective as tamoxifen as a first-line therapy for postmenopausal MBC (68). However, unlike third-generation AIs, it has shown no survival benefit in this setting (69).
Formestane has poor oral bioavailability. It is given at 250 mg IM every 2 weeks (70). With the development of third-generation oral AIs, formestane’s clinical use had decreased.
Side effects are similar to other AIs. Additionally, formestane administration has been associated with formation of sterile abscesses at the injection site.
Exemestane is a third-generation steroidal AI. It has been studied as second-line therapy in tamoxifen-resistant patients and in patients who have progressed on a nonsteroidal AI, such as anastrozole and letrozole (71–74). Kvinnsland et al. reported an overall response rate of 23%, with an additional 24% having stable disease at 24 weeks, in post-menopausal tamoxifen-resistant MBC (63). When compared with megestrol acetate in postmenopausal tamoxifen-resistant advanced breast cancer, exemestane showed an objective response rate of 15% versus 12% in the megestrol-treated group. Median survival and time to progression (20) vs 17 weeks) were longer in the exemestane group as well (75).
In the EORTC trial comparing tamoxifen 20 mg daily with exemestane 25 mg daily as a first-line therapy in postmenopausal MBC, exemestane had superior objective response rate of 46% versus 31% in the tamoxifen group (16,76). It also showed better median PFS of 9.9 versus 5.8 months, but this did not render an OS benefit.
Exemestane has been reported to be effective in patients with primary tamoxifen resistance and in those who progress on nonsteroidal AI therapy (63,64,71–75,77).
Table 3 presents a summary of clinical data about third-generation AIs as first-line treatment for hormone receptor-positive MBC.
Side Effects of AIs
Common adverse reactions of selective AI therapy include hot flashes (12–36%), vasodilatation (25–36%), arthritis (17%), arthralgias (15%), asthenia (16–19%), bone pain (11%), myalgias (6%), nausea (19%), vomiting (8%), hypertension (5–13%), hypercholesterolemia (9%), angina (2–12%), depression (13%), peripheral edema (8%), rash (8%), increased incidence of bone loss/ osteoporosis (11%), and related fractures (10%). Osteoporosis may require concurrent treatment with bisphosphonates. Depending on the severity, hot flashes, arthralgias, and/or rapidly progressive bone demineralization may require discontinuation of AI therapy.
Although relatively uncommon, anaphylaxis (<61%), angioedema (<1%), cerebrovascualar acci-dent (2%), myocardial ischemia (1%), venous thrombosis and pulmonary embolism (<1%), and hepatitis with or without jaundice (<1%) have been reported.
TABLE 3
Third-generation aromatase inhibitors (available in the United States) as first-line therapy
Agent | Dosage | Objective Response Rate (CR + PR)a | Median Survival |
Anastrozole | 1 mg PO daily | 21 vs 17% with tamoxifen | Median TTP of 11.1 months vs 5.6 months with tamoxifen (two-sided P = .005) (12) |
Letrozole | 2.5 mg PO daily | 24 vs 16% with tamoxifen | 34 vs 30 months with tamoxifen (not statistically significant) (59) |
Exemestane | 25 mg PO daily | 46 vs 31% with tamoxifen | > |
 POSTMENOPAUSAL MBC
POSTMENOPAUSAL MBC


