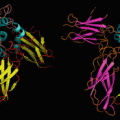and Winfried G. Rossmanith2
(1)
Lenzkirch, Germany
(2)
Ettlingen, Germany
Although rhythms during hormonal release have fascinated endocrinologists for a long time, many questions—especially regarding their physiological purpose—have not been answered. The origin and relevance of secretory episodes (pulses) from the hypothalamus and pituitary in their 1–3-h intervals are not known. The mechanisms, for example, of regular gonadotropin-releasing hormone (GnRH) release during the fertile years in men and women as well as in many animals defy analysis. Many elements, however, have been defined which determine in isolated neurons or larger tissue aggregates the pulse frequency and pulse amplitude .
Secretory maxima can be observed from active organs. Peak levels , with their rhythmic repetitions, are analyzed in serum . Concentration maxima (peaks or pulses) alternate periodically with minimal secretion (nadirs). Short intervals are defined as peaks with spans of 2–3 h or shorter. Serum-level oscillations during a whole day (circadian pulses), for example, are exemplified by cortisol, with a peak in the morning and a nadir in the evening. Furthermore, serum concentrations of growth hormone or melatonin increase during the night and are dependent on as well as independent of sleep. The annual or seasonal animal fertility cycles and hibernation phases have ultralong rhythms. Complex networks of regulations are at the origin of these periodic changes, and hormones are major parameters. The analysis of the causal zeitgeber of these rhythms is beyond the grasp of endocrinology.
Circadian light–dark cycles and annual temperature oscillation are periodic parameters which act extrinsically on any organism. There is, however, in plants and animals a conserved pacemaking mechanism with similar principles in protozoans and metazoans.
12.1 A Universal Pacemaker
The control of the generation and degradation of seven proteins is sufficient to generate a rhythm of about 24 h (circadian). The major functions of these proteins are described in Table 12.1. Decisive is the feedback inhibition exerted by the protein period (PER) on the transcription of PER RNA and thus on its own synthesis. Further important aspects of this autonomous oscillation are as follows:
Phosphorylation of PER by casein kinase 1 epsilon (CK1ε) , which is required for PER to get into the cellular nucleus
Binding of phosphorylated PER to cryptochrome (CRY ), allowing transport of the PER–CRY dimer into the nucleus
Constitutive transcriptional activation of PER by promoter binding of the dimer formed between brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like protein 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK)
Blocking of any PER transcription by binding of PER–CRY to promoter-bound BMAL1–CLOCK
Ubiquitin-induced protein degradation—for example, by modification with ubiquitin and subsequent protease digestion in proteasomes
Table 12.1
Proteins of the zeitgeber
Name/OMIM entrya | Function/comment |
|---|---|
BMAL1/602550 | BMAL1 dimerizes to CLOCK. The dimer interacts with E-box es: CACGTG DNA motifs with five E-boxes in the PER promoter and one in the CRY promoter. Dimer binding to the E-box activates transcription of PER and CRY. BMAL1–CLOCK levels are enough to permanently cover all E-boxes. By binding of PER–CRY dimer to a single E-box, PER and CRY transcription is inhibited. ARNT is the nuclear transport mediator for the aryl hydrocarbon receptor (with which dioxin or DDT interacts) and which stimulates CYP1A1 and activation of other CYP enzymes in response to foreign substances. BMAL1 is very similar to ARNT. BMAL1 has bHLH and PAS domains |
CLOCK/601851 | CLOCK dimerizes to BMAL1 and binds as a dimer to E-box es (see above). In 1997, 25 years after the discovery that the supraoptic nucleus plays a role in circadian oscillations, the mouse CLOCK protein was described by two groups as required for maintenance of circadian rhythms. CLOCK has, like PER and BMAL1, bHLH and PAS domains |
PER1/602260 | Transcription of PER b and the presence of PER obey a 24-h rhythm. PER is transcribed when no PER protein is present. After the cytosolic synthesis of PER, it is phosphorylated by CK1ε and CK2ε. Phosphorylated PER is co-transported with CRY into the nucleus and blocks the transcription of its own gene: classic feedback inhibition . PER proteins consist of bHLH and PAS domains, the former for DNA interaction (see Fig. 12.1), the latter for dimerization |
CRY1/601933 | CRY binds cytosolically phosphorylated PER. Thereafter, the complex enters the nucleus. Therein by it binding to BMAL1–CLOCK dimers, PER transcription is blocked. Binding by CRY makes the protein TIM (for “timeless”) ready for degradation in proteasomes. Photolyases help to eliminate UV defects from DNA. They contain flavin adenine dinucleotide and pterin groups. CRY proteins are such photolyases; their role, however, appears to be to act as blue light photoreceptors. For their involvement in the circadian rhythm, the photolyase function is not important |
CK1ε /600863 | CK1ε cytosolically associates with PER and phosphorylates PER several times. This phosphorylated PER then couples to CRY and enters the nucleus. Casein kinases are widely distributed. They phosphorylate serine or threonine residues. CK1ε mutations change the circadian rhythm of flies and hamsters. The hamster mutant tau inhibits autophosphorylation of CK1ε and reduces binding to PER. Thus, nuclear availability of PER is diminished and the circadian rhythm is changed. The same applies for CK2ε |
Translation of proteins other than PER —CRY , BMAL1 , and CLOCK also occurs periodically, potentially blocked by CRY . Forger and Peskin (2003) developed a model from biochemical data and some assumptions which simulates a periodic almost 24-h autonomous oscillation. Their model takes into account the seven proteins, the transcriptional activity, nuclear–cytosol transport, translation, phosphorylation, cytosol–nuclear transport, and ubiquitin-mediated degradation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree