Endocrine Disease: Diabetes Mellitus
Nutrition Concepts by Franz, Inc., Minneapolis, MN
Introduction
Diabetes mellitus is manifested in three primary forms: type 1 diabetes, type 2 diabetes, and gestational diabetes mellitus (GDM). Diabetes is a group of diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Pathogenic processes involved in the development of diabetes range from autoimmune destruction of the beta cells of the pancreas with resultant insulin deficiency – type 1 diabetes – to abnormalities that result in resistance to insulin action and an inadequate compensatory insulin secretory response – type 2 diabetes.
Without sufficient insulin, hyperglycemia occurs, resulting in acute and long-term microvascular and macrovascular complications. Mild hypoglycemia may be inconvenient or frightening to patients with diabetes; more severe hypoglycemia can cause acute harm to the persons with diabetes or others, if it causes falls, motor vehicle accidents, or other injury. The stress of illness, trauma, and/or surgery frequently compromise glycemic control and may result in acute, life-threatening consequences of uncontrolled hyperglycemia such as ketoacidosis and non-ketotic hyperosmolar syndrome. Long-term complications of hyperglycemia include retinopathy, nephropathy, and peripheral and autonomic neuropathy. Patients with diabetes are also at high risk for atherosclerotic, cardiovascular, peripheral arterial, and cerebrovascular disease. Hypertension and lipoprotein abnormalities are other common problems. Therefore, it is essential that effective therapy to achieve normal glycemia levels be initiated early to prevent the deleterious effects of hyperglycemia. Treatment for lipid disorders and hypertension is also essential.
Prevalence
In the United States, 25.8 million people of all ages are reported to have diabetes; 8.3 percent of the population. Of these 18.8 million who are diagnosed, 7.0 million remain undiagnosed. Diabetes prevalence increases with increasing age, affecting 10.9 million or 26.9 percent of those 65 years of age or older and is particularly prevalent in ethnic populations, including African-American, Latino, Native American, Asian American, and Pacific Islander. Approximately 215,000 youth under 20 years of age had diabetes (type 1 or type 2) in the United States in 2010. Among children with newly diagnosed diabetes, the prevalence of type 2 diabetes increased from less than 4 percent prior to 1990 to as high as 45 percent in certain racial/ethnic groups in recent years.
An estimated 79 million or 35 percent of United States adults who, based on fasting glucose or hemoglobin A1c (A1C) levels, are reported to have prediabetes are at high risk for developing type 2 diabetes, heart disease, and stroke. In adults aged 65 years or older, 50 percent have prediabetes. Lifestyle prevention strategies for people with prediabetes including weight loss and physical activity increases can prevent or delay the onset of type 2 diabetes and in some cases return blood glucose levels to normal (Chapter 1: Case 1; Chapter 9: Case 2).
Diagnosis of Diabetes Mellitus or Prediabetes
Prior to 2009, the diagnosis of diabetes was based on plasma glucose criteria, either the fasting plasma glucose (FPG) or the 2-hour value in the 75-g oral glucose tolerance test (OGTT). Beginning in 2010, the American Diabetes Association (ADA) also recommended the use of the A1C test to diagnose diabetes. The A1C test has several advantages to FPG and the OGTT, including greater convenience (since fasting is not required), greater preanalytical glucose stability, and less day-to-day variations due to illness or stress. Any of the following diagnostic criteria for diabetes can be used:
- A1C ≥6.5 percent;* or
- FPG ≥126 mg/dL (7.0 mmol/L);* or
- 2-hour PG ≥200 mg/dL (11.1 mmol/L during an OGTT using a glucose load containing the equivalent of 75 g of anhydrous glucose dissolved in water;* or
- In patients with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥200 mg/dL (11.1 mmol/L).*
*In the absence of unequivocal hyperglycemia, results should be confirmed by repeat testing.
Hyperglycemia that does not meet diagnostic criteria for diabetes is categorized as either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT). Both IFG and IGT are categories of increased risk for diabetes (prediabetes): *
- FPG 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L) (IFG); or
- 2-hour plasma glucose in the 75-g OGTT 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) (IGT); or
- A1C 5.7 to 6.4 percent.
*For all three tests, risk is continuous, extending below the lower limit of the range and becoming disproportionately grater at higher ends of the range.
Testing for Prediabetes and Diabetes
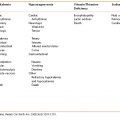
Unfortunately, type 2 diabetes is frequently not diagnosed until complications appear. Therefore, testing to detect prediabetes and type 2 diabetes in asymptomatic adults is important. There is a long presymptomatic phase before the diagnosis of type 2 diabetes is usually made and effective interventions can be utilized to prevent progression from prediabetes to diabetes and to reduce the risk of complications. Testing should be considered in all adults at any age with a body mass index (BMI) ≥25 kg/m2 and who have one or more of the known risk factors for diabetes listed in Table 8-1. In those without risk factors, testing should begin no later than age 45. If tests are normal, testing should be repeated at 3-year intervals, with considerations of more frequent testing depending on initial results and risk status (e.g., those with prediabetes should be tested yearly).
Table 8-1 Risk Factors for Development of Diabetes
Source: Adapted from American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 2014;37(Suppl 1):S14–S80.
|
Children and adolescents who are overweight (BMI >85th percentile for age and sex, weight for height >85th percentile, or weight >120 percent of ideal for height) and who have two or more additional risk factors for diabetes (listed below) should also be tested at age 10 years or at onset of puberty, if puberty occurs at a younger age. If tests are normal, testing should be repeated every 3 years. Risk factors include:
- Family history of type 2 diabetes in first- or second-degree relative.
- Race/ethnicity (Native American, African–American, Latino, Asian–American, Pacific Islander).
- Signs of insulin resistance or conditions associated with insulin resistance including acanthosis nigricans (gray-brown skin, pigmentations) hypertension, dyslipidemia, polycystic ovary syndrome, or small-for-gestational-age birth weight.
- Maternal history of diabetes or GDM during the child’s gestation.
Pathophysiology of Diabetes
Type 1 Diabetes
Type 1 diabetes accounts for 5 to 10 percent of all diagnosed cases of diabetes. The primary defect results from a cellular-mediated autoimmune destruction of pancreatic beta-cells leading to absolute insulin deficiency (insulinopenia), as well as hyperglycemia, polyuria, polydipsia, weight loss, dehydration, electrolyte disturbance, and ketoacidosis. The capacity of a healthy pancreas to secrete insulin is far in excess of what is needed normally; therefore, the clinical onset of diabetes may be preceded by an extensive asymptomatic period (months to years), during which beta cells are undergoing gradual destruction. Persons with type 1 diabetes are dependent on exogenous insulin to prevent ketoacidosis and death. Although it can occur at any age, most cases are diagnosed in people younger than age 30 years of age, with peak incidence between 10 to 12 years in girls and 12 to 14 years in boys.
Type 1 diabetes is a result of a genetic predisposition combined with the autoimmune destruction of the islet beta cells. At diagnosis, 85 to 90 percent of persons with type 1 diabetes have one or more circulating autoantibodies. Antibodies identified as contributing to the destruction of beta cells are (1) islet cell autoantibodies (ICAs); (2) insulin autoantibodies (IAAs), which may occur in persons who have never received insulin therapy; (3) autoantibodies to glutamic acid decarboxylase (GAD65), a protein on the surface of beta cells (GAD autoantibodies appear to provoke an attack by killer T lymphocytes, which may be what destroys the beta cells in diabetes); and (4) autoantibodies to the tyrosine phosphatases IA-2 and IA-2β. Type 1 diabetes also has strong HLA association, with linkage to the DGA and DQB genes, and is influenced by the DRB genes. These HLA-DR/DQ alleles can be either predisposing or protective.
Frequently, after diagnosis and the correction of hyperglycemia, metabolic acidosis, and ketoacidosis, endogenous insulin secretion recovers. During this “honeymoon phase,” exogenous insulin requirements decrease dramatically. However, the need for exogenous insulin is inevitable, and within 8 to 10 years after clinical onset, beta-cell loss is complete and insulin deficiency is absolute. The rate of beta-cell destruction is quite variable, being rapid in some individuals, mainly infants and children, and slow in others, mainly adults.
Type 2 Diabetes
Type 2 diabetes accounts for 90 to 95 percent of all diagnosed cases of diabetes and is a progressive disease that is often present long before it is diagnosed. Although approximately 50 percent of men and 70 percent of women are obese at the time of diagnosis, type 2 diabetes also occurs in non-obese individuals, especially in older adults. An affected individual may or may not experience the classic symptoms of uncontrolled diabetes, and they are not prone to develop ketoacidosis. Insulin resistance begins and progresses for many years before the development of diabetes, but impaired beta cell insulin secretory function must be present before hyperglycemia manifests. By the time diabetes is diagnosed, the individual has lost as much as 50 percent of beta cell function.
In type 2 diabetes, the normal biphasic insulin response to glucose is altered, resulting in postprandial hyperglycemia. The inadequate first-phase insulin response is also unable to suppress pancreatic alpha cell glucagon secretion, resulting in glucagon hypersecretion, which leads to an increase in hepatic glucose production and fasting hyperglycemia. The other major metabolic abnormality is a decrease in the ability of insulin to act on target tissues: muscles, liver, and fat cells. Compounding the problem is glucotoxicity, a deleterious effect of hyperglycemia – on both insulin sensitivity and insulin secretion; hence, the importance of achieving near-euglycemia in persons with type 2 diabetes.
Insulin resistance is also demonstrated in adipocytes, leading to lipolysis and an elevation in circulating free fatty acids. Increased free fatty acids cause a further decrease in insulin sensitivity at the cellular level, impair insulin secretion, and augment hepatic glucose production (lipotoxicity). All these defects (cellular, hepatic, and beta-cell) contribute to the development and progression of type 2 diabetes.
As type 2 diabetes progresses, insulin production progressively declines. Therefore, patients with diabetes typically require more medication(s) over time and eventually exogenous insulin will be required. This is not a “diet” or medication failure, but rather a failure of beta cell function.
Nutrition Therapy for the Prevention of Diabetes
The increase in diabetes worldwide has made prevention of type 2 diabetes a high priority. Individuals with prediabetes are at high risk for the development of diabetes and cardiovascular disease. Large randomized controlled trials in individuals with prediabetes have repeatedly shown that lifestyle interventions are effective in all ethnic groups, different age-groups, and various social and cultural settings worldwide. Modest weight loss (5 to 7 percent of body weight) and moderate physical activity (equivalent to 30 minutes brisk walking on most days of the week) are reported to decrease the risk of developing diabetes by 29 to 67 percent and/or delaying the onset of type 2 diabetes for at least 10 years.
Several trials have tested how efficacious drugs (i.e., metformin, acarbose, orlistat, rosiglitazone) would be in the prevention of diabetes. Each decreased the incident of diabetes to various degrees. Based on cost and side effects, the ADArecommends that only metformin be used, in combination with lifestyle counseling in those with IGT, IFG, or an A1C of 5.7 to 6.4 percent, and especially for those with BMI >35 kg/m2, aged <60 years, or women with prior GDM.
Based on the evidence, the following are lifestyle recommendations for the prevention of diabetes:
- Structured programs that emphasize lifestyle changes including education, reduced fat and energy intake, regular physical activity, and regular participant contact can produce a long-term weight loss of 5 to 7 percent of starting weight and reduce the risk of developing diabetes and are therefore recommended.
- Engaging in regular physical activity (150 min/week) will decrease risk of developing type 2 diabetes. Regular physical activity reduces insulin resistance, independent of weight loss, and while initial weight loss may be achieved through restriction of energy intake alone, it is unlikely that weight loss maintenance can be achieved without regular physical activity.
- Consuming at least 14 g fiber/1000 kcal per day and foods containing whole grains (at least one-half of grain intake).
- Limiting intake of sugar-sweetened beverages.
- Replacing saturated fatty acids with monounsaturated fatty acids or polyunsaturated fatty acids for improved insulin resistance and decreased risk of type 2 diabetes.
- Limiting alcohol to one to two alcoholic drinks per day is associated with a lower incidence of type 2 diabetes likely resulting from increased insulin sensitivity. However, the data do not support recommending alcohol use to people who do not currently drink and abstinence is recommended for people with risks related to alcohol consumption.
- Encouraging a Mediterranean-style eating pattern.
Treatment of Diabetes
Diabetes is a chronic disease that requires lifetime changes in lifestyle. The management of diabetes includes appropriate medical nutrition therapy, regular physical activity, self-management education, and medications. An important goal is to provide the individual with the necessary tools to achieve the best possible control of glycemia, lipids, and blood pressure to prevent, delay, or arrest the microvascular and macrovascular complications of diabetes while minimizing hypoglycemia and promoting weight loss and preventing weight gain.
Optimal control of diabetes requires the restoration of normal carbohydrate, protein, and fat metabolism. Insulin is both anticatabolic and anabolic and facilitates cellular glucose transport. In general, the counterregulatory hormones – glucagon, growth hormone, cortisol, epinephrine, and norepinephrine – have the opposite effect of insulin. In addition, incretin hormones (glucagon-like peptide 1 [GLP-1] and glucose-dependent insulinotrophic polypepetide, also called gastric inhibitory peptide [GIP]) are released from the gastrointestinal tract after food ingestion causing an “anticipatory” increase in insulin in preparation for the glucose and amino acids to be absorbed from the food.
Monitoring of Metabolic Outcomes
Glycemic control can be assessed by self-monitoring of blood glucose (SMBG), interstitial glucose by continuous glucose monitoring (CGM), or A1C measurement. Persons with diabetes can use SMBG to determine the impact that food choices and physical activity have on blood glucose levels and to make adjustments in lifestyle and medications required to achieve glycemic goals. The ADA recommends that patients on multiple-dose insulin (MDI) or insulin pump therapy do SMBG prior to meals and snacks, occasionally postprandially, at bedtime, and prior to exercise, or when they suspect low blood glucose, after treating low blood glucose until they are normoglycemic, and prior to critical tasks such as driving. For persons on non-insulin therapy, SMBG should be done sufficiently to facilitate reaching glucose goals. The accuracy of SMBG is instrument- and user-dependent and monitoring techniques must be evaluated on a regular basis. Patients also should be taught how to use the data to adjust food intake, exercise, or medications to achieve glycemic goals.
CGM measures interstitial fluid glucose (which correlates with blood glucose) in a continuous and minimally invasive manner. Continuous glucose sensors have alarms for hypo- and hyperglycemia and small studies have shown the use of CGM to decrease the average time patients spend in hypo- and hyperglycemic ranges. CGM used with an intensive insulin regimen or insulin pump can be a useful tool to lower A1C in selected adults (aged ≥25 years) with type 1 diabetes.
Complementing day-to-day testing are measurements of glycosylated hemoglobin (simplified as A1C) reflecting a weighted average of plasma glucose over the preceding 6 to 8 weeks, and thus reflecting long-term glycemic control. When hemoglobin and other proteins are exposed to glucose, the glucose becomes attached to the protein in a slow, non-enzymatic, and concentration-dependent manner. The A1C test should be done at least two times a year in patients who are meeting treatment goals and who have stable glycemic control. The A1C test should be done quarterly in patients whose therapy has changed or who are not meeting glycemic goals. Lowering A1C to around 7 percent or below has been shown to reduce microvascular complications of diabetes and if implemented soon after the diagnosis of diabetes is associated with long-term reduction in macrovascular disease. Therefore, the ADA recommends that a reasonable A1C goal for many non-pregnant adults is <7 percent. A lower A1C goal (such as <6.5 percent) might be reasonable for selected individual patients, if this can be achieved without significant hypoglycemia or adverse effects of treatment. Conversely, a less stringent goal (such as <8 percent) may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, and advanced complications.
Lipid levels and blood pressure should also be monitored. In most adult patients with diabetes, a fasting lipid profile should be measured at least annually. Blood pressure should be measured at every routine visit. The ADA glycemic, lipid, and blood pressure goals are listed in Table 8-2.
Table 8-2 Clinical Goals for Diabetes Therapy
Source: Adapted from American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 2014;37(Suppl 1):S14–S80.
| Recommendations for Many Non-Pregnant Adults with Diabetes | Goal |
|---|---|
| Glycemic | |
| A1C | <7.0%* |
| Preprandial capillary plasma glucose | 70–130 mg/dL* (3.9–7.2 mmol/L) |
| Peak postprandial capillary plasma glucose† | <180 mg/dL* (<10.0 mmol/L) |
| Lipids | |
| LDL-C (without overt CVD) | <100 mg/dL (<2.6 mmol/L) |
| LDL-C (with overt CVD) | <70 mg/dL (<1.8 mmol/L |
| Triglycerides | <150 mg/dL (<1.7 mmol/L) |
| HDL-C | >40 mg/dL (>1.0 mmol/L) in men |
| >50 mg/dL (>1.3 mmol/L) in women | |
| Blood pressure | |
| Blood pressure | <140/80 mm Hg |
*Goals should be individualized based on duration of diabetes, age/life expectancy, comorbid condition, hypoglycemia unawareness.
† Postprandial glucose measurements should be made 1–2 h after the beginning of the meal, generally peak levels in persons with diabetes.
Medical Nutrition Therapy
A healthy eating pattern and regular physical activity are important goals of medical nutrition therapy for all individuals with prediabetes and diabetes. For individuals with diabetes, other goals of medical nutrition therapy (MNT) are to assist in:
- attaining individualized glycemic, lipid, and blood pressure goals.
- achieving and maintaining body weight goals.
- delaying and preventing complications of diabetes.
Clinical trials and outcome studies support MNT as effective in improving metabolic outcomes, such as blood glucose, A1C, lipids, blood pressure, weight, and/or quality of life in persons with diabetes. The Academy of Nutrition and Dietetics Evidence-Based Nutrition Practice Guidelines (EBNPG) for diabetes documented decreases in A1C of 1 to 2 percent (range: −0.5 to −2.6 percent) with MNT depending on the type and duration of diabetes and at what time point in the disease process interventions are implemented. These outcomes are similar to those from glucose-lowering medications. The evidence suggests that MNT is most effective at initial diagnosis, but is effective at any time during the disease process. Many different types of nutrition interventions are effective. Central to these interventions are multiple encounters to provide education and counseling initially and on a continued basis. These outcomes highlight the importance of the registered dietitian (RD) in determining the most effective nutrition intervention for each individual and coordinating care with a multidisciplinary team.
In studies done primarily in individuals without diabetes, cardioprotective MNT implemented by RDs resulted in a reduction of total cholesterol by 7 to 21 percent, LDL-cholesterol by 7 to 22 percent, and triglycerides 11 to 33 percent. In hypertensive patients who consume excessive sodium, reducing intake to approximately 2300 mg/day can lower systolic blood pressure by 2 to 8 mm Hg; implementing a DASH eating pattern can lower systolic blood pressure by 8 to 14 mm Hg (Chapter 6). It is important to remember that these results are achieved without any attendant risk of medication side-effects.
Results from nutrition interventions on glycemia, lipids, and blood pressure are generally known by 6 weeks to 3 months and an evaluation should be performed by the RD at this time. At 3 months, if patients have made the lifestyle changes they are able and willing to make, and if target goals have not been achieved, medication changes (or adjustments) are usually needed and should be recommended to achieve target goals.
Achieving nutrition-related objectives requires a multidisciplinary team effort that includes dietitians, diabetes educators, physicians, nurse practitioners, and the person with diabetes who must be involved in problem solving. A system of care that provides ongoing support and education is essential.
Prioritizing Nutrition Strategies for Diabetes
Historically, nutrition advice has been given to patients with diabetes, such as “don’t eat foods with sugar” or “go home and lose weight.” This advice has often been accompanied by a calorie-level “diet sheet” or a pamphlet or brochure with general guidelines. Patients often find such information difficult to understand and implement. To achieve positive outcomes, appropriate priorities should be set for each patient.
Type 1 Diabetes The first priority for persons requiring insulin therapy is to integrate an insulin regimen into the patient’s lifestyle. After an initial food/meal plan is determined (with the patient’s input), it should be reviewed with the health professional who is planning the insulin regimen. With the many insulin options now available, an insulin regimen can usually be developed that will conform to the patient’s preferred meal times and food choices. Flexible insulin regimens using basal (background) insulin and bolus (mealtime) insulin or insulin pumps give the patient freedom in timing and composition of meals and are the preferred mode of therapy to maximize blood glucose control and minimize complications.
The total amount of carbohydrate in the meal (and snacks, if desired) is the major determinant of the bolus rapid-acting insulin dose and post-prandial glucose response. After determining the amount of insulin required to cover the patient’s usual meal carbohydrate, patients can be taught how to adjust bolus insulin doses based on the amount of carbohydrate they are planning to eat (insulin-to-carbohydrate ratio). For persons receiving fixed insulin regimens and not adjusting mealtime insulin doses, consistency of day-to-day carbohydrate amounts at meals is important.
Type 2 Diabetes Previously, nutrition advice focused on losing weight and avoiding sugars. Today, the focus of MNT for type 2 diabetes is to implement lifestyle strategies that will assist in improving glycemia, dyslipidemia, and blood pressure. Since many persons with type 2 diabetes are insulin resistant and overweight, MNT often begins with lifestyle strategies that reduce energy intake and increase energy expenditure through physical activity. However, many individuals have already tried unsuccessfully to lose weight and it is important to note that other lifestyle strategies, even without weight loss, can improve glycemia. Effective nutrition interventions include reduced energy/fat intake, individualized MNT, portion control, healthy food choices, and carbohydrate counting. A consistent and important theme for individuals with type 2 diabetes is reduced energy intake, which may or may not lead to substantial weight loss, but consistently improves glycemic control.
Modest amounts of weight loss and regular physical activity have been proven to prevent the progression of prediabetes to type 2 diabetes. Weight loss, especially of intra-abdominal fat, reduces insulin resistance; however, as individuals move from being primarily insulin resistant to insulin deficiency, the role of weight loss becomes more controversial. At later stages of the disease when medications, including insulin, need to be combined with MNT, prevention of weight gain often becomes an issue, although glycemic control must take precedence over concern about weight. In weight loss studies in subjects with type 2 diabetes, weight loss seems to plateau at 6 months, and weight loss is more difficult to accomplish in people with diabetes.
Unfortunately, weight loss of 4.7 kg among patients with type 2 diabetes did not result in greater decreases in cardiovascular events compared to control, in spite of improved A1C, HDL, thyroid hormone, and blood pressure.
In addition, a recent systematic review of weight-loss interventions in overweight/obese adults with type 2 diabetes demonstrated no differences by types of intervention and only a non-significant impact on A1C. This may be because most of the trials failed to achieve a weight loss of 5 percent or greater from baseline. Thus, the majority of the trials could not achieve weight losses that seem to be necessary to produce beneficial metabolic outcomes. A weight loss of >6 kg (7 to 8.5 percent), regular physical activity, and frequent contact with RDs appears necessary for consistent beneficial metabolic outcomes. How to achieve this weight loss in randomized trials remains unclear.
Macronutrient Recommendations
Numerous studies have attempted to identify the optimal mix of macronutrients to guide eating patterns for people with diabetes. The ADA systematic review of macronutrients concluded that there is not one effective mix that applies broadly, and that the mix of carbohydrate, protein, and fat should be adjusted to meet the metabolic goals and individual preferences of each individual.
Carbohydrate Because carbohydrate and adequacy of insulin determine post-prandial glucose response, it is addressed first. With the continued popularity of low-carbohydrate diets, it should be remembered that foods containing carbohydrates – fruits, vegetables, whole grains, legumes, and low fat-milk – are important components of a healthy diet and should be included in an eating plan for persons with diabetes. Furthermore, the majority of people with diabetes do not eat a high (or low) carbohydrate diet. Both people with type 1 and type 2 diabetes report eating moderate amounts of carbohydrate. Vegans or vegetarians are perhaps the primary people with diabetes who tend to eat a higher carbohydrate diet (∼65 to 75 percent of total energy), which is reported to improve glycemic control as well as serum lipids and blood pressure.
Observational studies in persons with type 1 and type 2 diabetes report A1C benefits from a higher carbohydrate, lower-fat eating pattern. It is suggested, however, that the carbohydrate content may be less important than the total and saturated fat content. Higher fat, especially saturated fat, intake in numerous studies has been shown to contribute to an increase in insulin resistance. High-fat meals have been shown to interfere with insulin signaling, whereas lower fat diets improve insulin sensitivity.
In clinical trials both high- or low-carbohydrate eating patterns lead to similar improvements in A1C and body weight. The total energy intake appears to be of more importance that the type or amount of carbohydrate. Therefore, it seems appropriate to recommend an eating pattern with moderate amounts of fruits, vegetables, whole grains, legumes, and low-fat dairy foods – all components of a healthy eating pattern.
The glycemic index (GI) measures the relative area under the glucose curve of 50 g of digestible carbohydrate compared with 50 g of either glucose or bread. It does not measure how rapidly blood glucose levels increase after eating carbohydrate-containing foods (“fast-acting” carbohydrates), which is a common misimpression given to the public. A major problem with the GI is the variability of responses to carbohydrate-containing foods among individuals. Short-term studies comparing high- versus low-GI diets report mixed effects on A1C levels. The Canadian Trial of Carbohydrate in Diabetes, a 1-year study comparing low or high GI diets, reported no significant difference in A1C or lipids by altering the GI or the amount of carbohydrate. An ADA systematic review concluded: “In general, there is little difference in glycemic control and cardiovascular disease risk factors between low-GI and high-GI diets.” Furthermore, as with carbohydrate, most individuals with diabetes seem to consume a moderate GI diet and it is unknown whether reducing the usual GI by a few units will result in improved long-term glycemic control.
All persons with diabetes can, however, benefit from basic information about carbohydrates: what foods contain carbohydrate (starches, fruit, starchy vegetables, milk, sweets and desserts; one average serving is equivalent to 15 g) and how many servings to select for meals (and snacks if desired). For all, monitoring total carbohydrate intake either by carbohydrate counting, food selection, or experience-based estimation is a key strategy in achieving glycemic control. The following are recommendations for carbohydrates:
- In persons on MNT alone, glucose-lowering medications, or fixed insulin doses, meal (and snack) carbohydrate intake should be kept consistent on a day-to-day basis, as consistency has been shown to result in improved glycemic control. For persons with type 2 diabetes total energy intake is important and therefore careful attention to portion sizes is critical.
- In persons with type 1 or type 2 diabetes who adjust their mealtime insulin or who are on insulin pump therapy, insulin doses should be adjusted to match carbohydrate intake (insulin-to-carbohydrate ratios).
- Recommendations for fiber intake are similar to recommendations for the general public (DRI: 14 g/1000 kcal per day). Diets containing 44 to 50 g of fiber per day are reported to improve glycemic control; however, fiber intakes up to 24 g/day have not shown beneficial effects on glycemia. Diets high in total and soluble fiber, as part of cardioprotective nutrition therapy, have been shown to reduce total cholesterol by 2 to 3 percent and LDL-C up to 7 percent.
- Whole-grain foods contain fiber, vitamins, minerals, phenolic compounds, and phytoestrogens lower serum lipids and blood pressure, improve glucose and insulin metabolism and endothelial function, and alleviate oxidative stress and inflammation in the general population. At least half of recommended grain intake should be whole grains.
- If persons with diabetes choose to eat foods containing sucrose, the sucrose-containing foods should be substituted for other carbohydrate foods. Sucrose intakes of 10 to 35 percent of total energy intake do not have a negative effect on glycemic or lipid responses when substituted for isocaloric amounts of starch.
- Non-nutritive sweeteners and sugar alcohols are safe when consumed within the accepted daily intake levels established by the Food and Drug Administration. However, some of these products may contain energy and carbohydrate from other sources.
- Eating a minimum of 5 servings of fruits and vegetables daily is recommended for both prevention and treatment of high blood pressure.
- Whole-grain foods contain fiber, vitamins, minerals, phenolic compounds, and phytoestrogens lower serum lipids and blood pressure, improve glucose and insulin metabolism and endothelial function, and alleviate oxidative stress and inflammation in the general population. At least half of recommended grain intake should be whole grains.
Protein Aside from sugars, protein is probably the most misunderstood nutrient with inaccurate advice frequently given to persons with diabetes. Although non-essential amino acids serve as substrates for gluconeogenesis, in subjects with controlled diabetes, this glucose does not enter the general circulation. Ingestion of protein results in acute insulin and glucagon responses with minimal, if any glucose or lipid response. Protein also does not slow the absorption of carbohydrate, but because it can increase acute insulin responses without increasing glucose concentrations, it should not be used to treat acute hypoglycemia or to prevent overnight hypoglycemia (e.g., by adding protein to bedtime snacks).
There is no evidence to suggest that usual intake of protein (15 to 20 percent of energy intake) be changed in persons who do not have renal disease. In persons with diabetic kidney disease (DKD), (either micro- or macroalbuminuria), lower protein diets (achieved average of 0.9 g/kg per day) versus usual protein diets (average 1.2 g/kg per day) do not significantly improve the rate of decline of glomerular filtration rates (GFR). Therefore, the ADA 2013 nutrition recommendations concluded that reducing the amount of dietary protein below the usual intake is not recommended because it does not alter glycemic measures, CVD risk measures, or the course of GFT. Therefore, the focus of MNT for persons with DKD should be on assisting with control of blood glucose levels and hypertension (Chapter 9: Renal Disease).
Dietary Fat A long-term high fat and high saturated fat diet are associated with an increase in insulin resistance. Limiting intake of saturated fatty acids to less than 7 percent of total energy, consuming minimal trans fatty acids, and dietary cholesterol less than 200 mg/day are recommended.
The ADA also recommends two or more servings of fish per week (with the exception of commercially fried fish filets). Plant sterol and stanols esters have also been shown to lower total and LDL-C in persons with type 2 diabetes and can be substituted for other fats in the diet, such as margarine or cream cheese. However, the ADA does not recommend omega-3 supplements for primary or secondary prevention of CVD as randomized controlled trials do not provide evidence for their effectiveness.
Micronutrient Recommendations
Vitamins and Minerals There currently is no evidence of benefit from vitamin or mineral supplementation in persons with diabetes who do not have underlying deficiencies. Routine supplementation with antioxidants is not advised because of lack of evidence of effectiveness and concerns related to long-term safety. There is also insufficient evidence to support the use of chromium, magnesium, and vitamin D to improve glycemic control in persons with diabetes.
Sodium For both normotensive and hypertensive individuals, a reduction in sodium intake lowers blood pressure. The recommendation for the general public to reduce sodium to <2300 mg/day is also appropriate for persons with diabetes. For persons with both diabetes and hypertension, further reduction in sodium intake should be individualized.
Alcohol Recommendations for alcohol intake are similar to those for the general public. If individuals choose to drink, alcoholic beverage consumption should be limited to an average of up to 2 drinks per day for men and an average of up to 1 drink per day for women. One drink is defined a 12 ounces beer, 5 ounces wine, or 1.5 ounces of distilled spirits, each of which contains approximately 15 g of alcohol. For individuals using insulin or insulin secretagogues, alcohol should be consumed with food to reduce the risk of hypoglycemia. Occasional use of alcoholic beverages should be considered an addition to the regular meal plan, and no food should be omitted.
Moderate amounts of alcohol, when ingested with food, have minimal acute or long-term effects on glucose and insulin concentrations in people with type 1 or type 2 diabetes. Moderate alcohol intake (1 to 2 drinks per day) is associated with a decreased risk of and mortality from cardiovascular disease (CVD). The type of alcohol-containing beverage does not make a difference. On the other hand, excessive amounts of alcohol (3 or more drinks per day), on a consistent basis, contribute to hyperglycemia, hypertension, cirrhosis, and other medical conditions.
Physical Activity
Physical activity should be an integral part of the treatment plan for persons with diabetes. Exercise helps improve insulin sensitivity, reduce cardiovascular risk factors, control weight, and improve well-being. People with diabetes can exercise safely. The exercise plan will vary depending on age, general health, and level of physical fitness. A minimum of 150 min/week of moderate intensity aerobic physical activity (50 to 70 percent of heart rate) is advised. In the absence of contraindications, resistance training three times per week is encouraged. Persons taking insulin or insulin secretagogues should monitor their blood glucose and take appropriate precautions to avoid hypoglycemia; carbohydrate should be eaten if pre-exercise glucose levels are less than 100 mg/dL (5.6 mmol/L).
Bariatric Surgery
Performing bariatric surgery in persons with diabetes continues to be controversial, especially in persons with a BMI of 30 to 35 kg/m2. The ADA recommendations state that bariatric surgery may be considered for adults with BMI >35 kg/m2 and type 2 diabetes, especially if the diabetes or associated co-morbidities are difficult to control with lifestyle and pharmacological therapy. A meta-analysis of 136 weight-loss surgery studies, including 22,094 individuals BMI ≥40 revealed an overall type 2 diabetes remission rate of 84 percent after Roux-en-Y gastric bypass and 48 percent after adjustable gastric banding 48 percent. Patients challenged with prediabetes may benefit the most since most studies report close to 100 percent prevention of progression to diabetes (Chapter 1: Case 2). The role of bariatric surgery in patients with type 2 diabetes and a BMI of 30 to 35 kg/m2 is under discussion In two recent reports, mildly to moderately obese persons with uncontrolled diabetes who underwent bariatric surgery had better short-term glucose control and weight loss than persons who received medications and lifestyle advice. But the surgery had potential complications and it is unknown whether the benefits of the surgical interventions extend beyond 1 to 2 years. Therefore, long-term benefits, cost-effectiveness, and risk of bariatric surgery in persons with type 2 diabetes require additional investigation.
Medications
Glucose-Lowering Medications If metabolic goals are not being met in persons with type 2 diabetes, there are now seven classes of oral medications as well as injectable medications, including insulin, which can be combined with nutrition therapy. This provides numerous options for achieving euglycemia in persons with type 2 diabetes. Metformin is usually the first line-drug, but many people benefit from taking two or more of the medications because each addresses a different problem. Such combination therapy is so common that a number of combination pills are also available. However, because of the progressive nature of type 2 diabetes, many individuals will also require insulin therapy alone or in combination with other agents to achieve glycemic control. Classes of glucose-lowering medications are:
- Amylin mimetics (pramlintide), which activate amylin receptors, there bydecreasing postmeal glucagon secretion and delaying gastric emptying;
- Alpha-glucosidase inhibitors (acarbose, miglitol), which work in the small intestine to inhibit α-glucosidase enzyme that digests carbohydrates, thereby delaying intestinal carbohydrate digestion/absorption and lowering postprandial glycemia;
- Biguanides (metformin), which suppress hepatic glucose production, lower insulin resistance, but do not stimulate insulin secretion;
- DPP-4 inhibitors (sitagliptin, saxagliptin, linagliptin), which inhibit dipeptidyl peptidase-4 (DDP-4) enzyme that degrades glucose dependent insulinotropic polypeptide (GIP) and glucose-like polypeptide (GLP), whose actions are to increase insulin secretion in the presence of elevated plasma glucose and to reduce postmeal glucagon secretion;
- GLP-1 receptor agonists (exenatide, exenatide extended release, liraglutide), which activate GLP-1 receptors, thereby enhancing insulin secretion in the presence of hyperglycemia, decreasing postmeal glucagon production, delaying gastric emptying, and may suppress appetite;
- Insulins (human NPH, human Regular, lispro, aspart, glulisine, glargine, detemir, premixed [several types]), which increase glucose disposal and decrease hepatic glucose production;
- Sodium-glucose transporter 2 (SGLT-2) inhibitors (canagliflozin, dapagliflozin) which cause glucose to be flushed out in the urine by blocking a transporter protein that returns glucose to the bloodstream after it is filtered through the kidneys;
- Meglitinides (nateglinide and repaglinide), which acutely promote insulin secretion and are taken at the start of a meal;
- Sulfonylureas (second-generation: glyburide/glibenclamide, glipizide, glimepiride), whose actions are to promote insulin secretion by the beta cells of the pancreas over longer periods of time;
- Thiazolidinediones (pioglitazone, rosiglitazone), which decrease insulin resistance in peripheral tissues and thus enhance the ability of muscle and adipose cells to take up glucose.
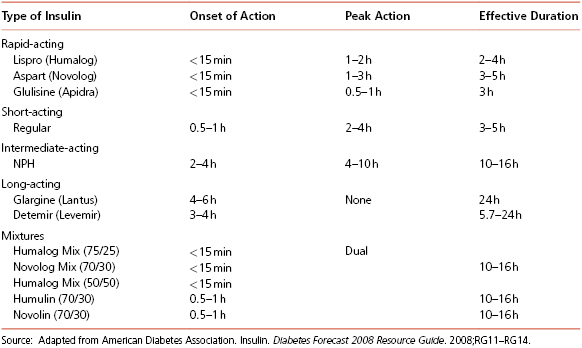
Insulin All persons with type 1 diabetes and many persons with type 2 diabetes who no longer produce adequate endogenous insulin need replacement of insulin that mimics normal insulin action. After eating, plasma glucose and insulin concentrations increase rapidly, peak in 30 to 60 minutes and return to basal concentrations within 2 to 3 hours in non-diabetics. To mimic this, rapid-acting insulin, such as lispro, aspart, or glulisine, is given at mealtime; doses are adjusted based on the amount of carbohydrate in the meal.
Basal or background insulin, such as determir, glargine, or NPH, is required in the post-absorptive state to restrain endogenous glucose output primarily from the liver and to limit lipolysis and excess flux of free fatty acids to the liver. Glargine and detemir are insulin analogs of 24-hour duration with no peak action time. They can be injected any time during the day, as long as they are taken around the same time each day, and cannot be mixed with other insulins. NPH is also occasionally used as background insulin but usually has to be given twice a day. The type and timing of insulin regimens should be individualized based on eating and exercise habits and blood glucose concentrations. There are also premixed insulins that are usually used in persons with type 2 diabetes, often when insulin is initiated.
Many patients find insulin pens to be a convenient way to inject their insulin doses. Insulin pens are available containing regular, NPH, lispro, aspart, glulisine, or 70/30 or 7/25 premixed insulin.
Insulin pump therapy delivers insulin in two ways: in a steady, measured, and continuous dose (the basal insulin), and as a surge (bolus) dose at mealtime. Insulin pumps can also deliver precise insulin doses for different times of day, which may be necessary to correct for situations such as the dawn phenomenon (increase in blood glucose level that occurs in the hours before and after waking). Pump therapy requires a committed and motivated person who is willing to do a minimum of four blood glucose tests per day, keep blood glucose and food records, and learn the technical features of pump usage.
Types of insulin and their action times are given in Table 8-3.
Table 8-3 Action Times of Human Insulin Preparations
Treatment of Hypoglycemia
Any carbohydrate-containing food will raise glucose levels, including glucose tablets, sucrose, juice, regular soda, or syrup. Glucose is the preferred treatment, and commercially available glucose tablets have the advantage of being premeasured to help prevent over-treatment. Treatment begins with 15 to 20 g of glucose and an initial response should be seen in approximately 10 to 20 minutes. Blood glucose should be evaluated again in approximately 60 minutes as additional treatment may be necessary. Adding protein has no benefit in treatment or in the prevention of subsequent hypoglycemia. Severe hypoglycemia (the individual is unable to ingest oral carbohydrate) requires administration of glucagon. For insulin users, prevention of hypoglycemia is a critical component of diabetes management.
Self-Management Education
For metabolic goals to be achieved there must be open communication and self-management education. With chronic illnesses such as diabetes, the role of healthcare providers shifts from providing direct medical care to facilitating self-management by individuals with diabetes and their families. Many healthcare providers choose to use a team approach with registered dietitians (as well as other team members) in their medical center or clinic or delegate the educational and skill-building components by referring to a registered dietitian and/or a diabetes education center.
It is reported that individuals who hold two important beliefs are more likely to engage in effectively self-management behaviors than are those who do not hold these beliefs: (1) consider diabetes to be serious and (2) believe that their own actions make a difference. An individual’s self-efficacy and self-confidence in making and maintaining a change are significant predictors of later adherence. A simple, but effective role that all healthcare providers can provide is to endorse and support lifestyle changes and to express confidence in the patient’s ability to make change.
Case 1 Type 1 Diabetes Mellitus and Diabetic Ketoacidosis
1 Albert Einstein College of Medicine, Bronx, NY
2 University of South Carolina School of Medicine Greenville, Greenville, SC
MN is a 33-year-old Hispanic woman with type 1 diabetes, which was diagnosed at the age of 30. She reports feeling lightheaded in the late afternoon and notes that she feels better after eating. MN also reports difficulty sleeping. Her blood glucose monitor log indicated from before breakfast to before dinner blood glucose. Tests range from 70 mg/dL and 150 mg/dL. MN is 5′4″ (163 cm) tall and currently weighs 132 pounds (60 kg). Recent assessment of her kidney function and an eye examination were normal.
Past Medical History
MN was diagnosed with type 1 diabetes 3 years ago after being brought to the emergency room by her mother in ketoacidosis. At the time of her diagnosis, MN recalls losing 9 pounds (4.1 kg) in 2 days after coming down with a cold and suffered from dizziness, fatigue, and frequent urination and experienced excess thirst and hunger.
Family History
MN’s mother developed type 1 diabetes in her late 30s and had poorly controlled diabetes until a hospitalization for a myocardial infarction six months ago. Her mother also has hypertension and microalbuminuria. Her paternal grandfather developed diabetes in his late 60s and was treated with oral medication.
Social History
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree