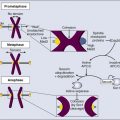
Manpreet K. Chadha and Donald L. Trump • Endocrine dysfunction may occur as a direct result of cancer or may be a consequence of cancer therapy (e.g., surgery, radiation, chemotherapy, biological agents, and hormone therapy). Endocrine dysfunction may be an intentional consequence or an adverse effect of antineoplastic therapy. • Hypopituitarism with clinically significant deficiencies of growth hormone, thyrotropin, gonadotropin, and corticotrophin may result from radiation (cranial or total body irradiation), surgery, or chemotherapy. • Thyroid dysfunction from neck irradiation, immune therapy (interleukin-2), and small molecule tyrosine kinase inhibitors such as sunitinib may result in either hyperthyroidism or hypothyroidism. • Gonadal dysfunction after surgery, radiotherapy, or chemotherapy results in disruption of puberty, infertility, and premature menopause. • Adrenal dysfunction from agents such as ketoconazole or aminoglutethimide may result in glucocorticoid or mineralocorticoid deficiency. • Pancreatitis and, occasionally, pancreatic exocrine or endocrine deficiencies may result from chemotherapy (l-asparaginase and streptozotocin). • A detailed history along with a complete physical examination is critical for diagnosis. Locations of primary and metastatic tumors along with past and current therapies are necessary elements of evaluation. • Signs and symptoms such as delayed or precocious puberty, fatigue, weight loss or gain, amenorrhea, orthostatic hypotension, hyperpigmentation, or electrolyte abnormalities should prompt consideration of unrecognized endocrine dysfunction. • When one hormonal deficiency is identified, others should be sought. • Basal serum hormone concentrations are usually sufficient; however, dynamic testing might be required to diagnose partial deficiencies. • Patients often have multiple, concurrent hormone deficiencies. Replacement therapy should be started as soon as possible. • Primary hypothyroidism is characterized by a low free thyroxine (T4) level and an elevated thyroid-stimulating hormone (TSH) level, whereas central hypothyroidism is associated with a low free T4 level and inappropriately normal or low TSH levels. Replacement with levothyroxine is indicated and is highly effective. • Hyperthyroidism is caused by increased T4 and/or T3 (triiodothyronine) levels with a low serum TSH level. Treatment options include surgery, radioiodine ablation, or antithyroid medications (e.g., propylthiouracil). Rarely, hyperthyroidism may be associated with production of TSH-like substances by germ cell or choriocarcinoma. Management in these instances involves thyroid suppression and treatment of the primary tumor. • Low- or high-dose corticotrophin testing can distinguish between central and primary causes of adrenal insufficiency. Acute adrenal insufficiency is a medical emergency and should be treated by immediate parenteral glucocorticoid replacement and supportive care. Chronic insufficiency is treated by oral glucocorticoid supplement with or without mineralocorticoid. Syndrome of Inappropriate Antidiuretic Hormone Secretion • Hyponatremia is classically associated with high-dose cyclophosphamide and vinca alkaloid administration. Measurement of serum and urine osmolality, renal function tests, and assessment of volume status of a patient are the key to diagnosis. Treatment involves fluid restriction and increased salt intake. Patients with refractory cases might need loop diuretics, doxycycline, or newer vasopressin receptor blockers. Endocrine dysfunction is an increasing cause of morbidity in patients with cancer. In the Childhood Cancer Survivor Study, one or more endocrine conditions were reported in 43% of childhood brain tumor survivors.1 Timely recognition and management of endocrine dysfunction are essential to prevent further morbidity and impairment of quality of life in patients with cancer. Box 61-1 outlines the causes of endocrine dysfunction among patients with cancer. Appropriate evaluation and treatment of common endocrinopathies are discussed in the latter sections of this chapter. A special section is included on surveillance of childhood cancer survivors for detection of late endocrine complications of various cancer therapies. A brief section is also included on endocrine adverse effects from newer therapeutic agents. Tumors of endocrine origin and neuroendocrine tumors are discussed in relevant sections of this textbook. Historically, surgery has been used as a means of disrupting normal endocrine function and was the first effective therapy for advanced breast or prostate cancers.2 Response rates of 15% to 30% were reported after hypophysectomy or adrenalectomy in patients with advanced breast cancer.3 However, these procedures resulted in significant morbidity, including hypoadrenalism and hypopituitarism, requiring lifelong replacement therapy. For premenopausal women, ovarian ablation by surgical oophorectomy remains a therapeutic option in metastatic and adjuvant settings. These surgical procedures have been largely supplanted by pharmacologic agents such as luteinizing hormone-releasing agonists along with aromatase inhibitors (that inhibit adrenal steroidogenesis) to attain functional castration.4 Orchiectomy, whether surgical or medical, is a critical therapeutic option for men with advanced prostate cancer.5 Similarly, resection of a tumor involving other endocrine glands may result in deficiencies of hormones secreted from these glands: thyroid (hypothyroidism), parathyroids (hypoparathyroidism), pancreas (diabetes mellitus), ovaries (hypogonadism), testes (hypogonadism), or adrenals (hypoadrenalism). Unilateral gland resection rarely results in noticeable hormone deficiencies. Extensive neck surgery and irradiation for advanced head and neck cancers may result in parathyroid hormone deficiency, which can be due to interference with the vascular supply of the parathyroids. Permanent hypoparathyroidism can result inadvertently from total thyroidectomy; the reported incidence is as high as 40%.6 Subtotal removal of parathyroid glands as a part of therapy for parathyroid hyperplasia can also cause hypoparathyroidism. It is often possible to preserve parathyroid function by careful surgical technique and/or by autotransplanting the parathyroid tissue to another part of the body. Endocrine organs may be intentionally or unavoidably exposed to ionizing radiation during treatment for malignancy, and high-dose radiation may result in endocrine dysfunction. Box 61-2 lists factors that are known to be associated with a high risk of endocrine dysfunction after radiation. Assessment of late effects of radiation may be difficult and subjective. Various groups have attempted to develop a scoring system to standardize toxicity reporting and description. The most accurate scale for grading radiation-induced effects on normal tissues, including to the hypothalamic-pituitary axis and thyroid, is LENT-SOMA (Late Effects on Normal Tissue—Subjective, Objective, Management and Analytic).7 These scales grade the radiation-induced adverse effects on organs exposed to irradiation by using criteria similar to the common toxicity criteria grading of adverse effects developed by the National Cancer Institute (http://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Anterior pituitary damage can result from irradiation of extracranial or primary brain tumors, especially those involving the pituitary. Total body irradiation as part of a bone marrow transplant preparative regimen and prophylactic cranial radiation in patients with acute lymphoblastic leukemia can also cause hypopituitarism.10–10 After curative irradiation for nasopharyngeal carcinoma, approximately 19% of patients have a deficiency in one or more anterior pituitary hormones as early as 2 years after therapy.10 Somatotrophs (cells that secrete growth hormone) are the most vulnerable to radiation damage; hence growth hormone deficiency (GHD) is the endocrine abnormality most commonly seen after cranial irradiation. GHD may occur in isolation after irradiation of the hypothalamic-pituitary axis even with doses less than 30 Gy. The clinical manifestations of GHD of reduction in growth velocity and short stature are most evident in the growing child.10 Although poor linear growth is very common in children with GHD, it is not universal or immediately apparent. Several studies suggest that the slowing of growth might not occur for the first year or two after the onset of GHD. In postpuberal individuals, GHD is associated with a decrease in muscle mass along with an increase in adiposity. The hypothalamic neurons secrete gonadotropin-releasing hormone (GnRH) in pulses that are necessary for normal secretion of gonadotropins from the pituitary. This GnRH pulse generation is affected differentially by the dose of radiation that is received. Higher dose irradiation (>30 Gy) is associated with delayed sexual maturation because of gonadotropin deficiency from damage to GnRH secretory neurons.11 Lower doses (<30 Gy) can result in precocious puberty equally in both sexes with a radiation dose of 30 to 50 Gy. Radiation-induced precocious puberty might be caused by damage to inhibitory GABAergic neurons, leading to disinhibition and premature activation of GnRH neurons.11 Deficiency in other pituitary hormones is less common. Littley and colleagues12 described 251 patients who had been treated for pituitary disease with external radiotherapy. Five years after completion of treatment, these investigators noted a 9% dose-related incidence of thyroid-stimulating hormone (TSH) deficiency at 20 Gy, which increased to 52% at 42 to 45 Gy. A similar trend in frequency of adrenocorticotrophic hormone (ACTH) deficiency was seen. Hyperprolactinemia related to damage to inhibitory neurons that control prolactin secretion can be seen after high-dose radiotherapy (>40 Gy); it has been described in both sexes and all age groups but is most common in young women. Hyperprolactinemia occurs with a described frequency ranging from 20% to 50% of patients with nasopharyngeal and brain irradiation.10 Hyperprolactinemia can cause delayed puberty in children, galactorrhea or amenorrhea in adult women, and decreased libido and impotence in men. Irradiation of the thyroid may produce hypothyroidism, Graves disease, silent thyroiditis, benign nodules, and thyroid cancers.13 Hancock and colleagues described a series of patients with thyroid dysfunction among patients treated with irradiation with or without chemotherapy for Hodgkin disease at Stanford University.14 Of 1787 patients, 1677 received irradiation to the thyroid. At 26 years of follow-up, the actuarial risk of thyroid disease was 67%. Hypothyroidism developed in the majority of the patients (47%). The risk of Graves disease was 7 to 20 times higher than that for healthy subjects. The risk of thyroid cancer was 15.6 times the expected risk for healthy subjects. These data remind clinicians to monitor thyroid function closely in patients who have been treated with upper mantle or cervical irradiation. Similar results were noted in the Childhood Cancer Survivor Study with an evaluable cohort of 1791 Hodgkin disease survivors (including 959 males). Among patients with Hodgkin disease, a 50% risk of hypothyroidism was found 20 years from the time of diagnosis in persons treated with 45 Gy or more.15 The total dose of irradiation received has been shown to correlate with the incidence of hypothyroidism in many studies.15–15 However, controversy exists regarding the effect of age and gender at the time of irradiation.15–15 Radiation-induced thyroid dysfunction is thought to be caused by damage to small thyroid vessels and to the glandular capsule. Histomorphologic features that are described in such patients include focal and irregular follicular hyperplasia, hyalinization, and fibrosis beneath the vascular endothelium; lymphocytic infiltration; single and multiple adenomas; and thyroid carcinomas.13,14 A rare complication of neck irradiation is acute radiation thyroiditis, which is more commonly associated with therapeutic doses of radioiodine for thyroid diseases. Patients typically have symptoms of fever, pain in the anterior cervical region, and transient hyperthyroidism. Hyperthyroidism with a clinical picture that resembles Graves disease may be seen after neck irradiation for Hodgkin disease.14 The incidence is uncertain because of the small number of cases reported. The clinical picture is characterized by diffuse thyroid enlargement, suppressed TSH, high levels of thyroid hormones, and development of thyroid autoantibodies. Ophthalmopathy, with or without overt hyperthyroidism, may be seen and is thought to be related to autoantibodies, similar to that seen in persons with classic Graves disease. Several studies link prior head and neck irradiation and hyperparathyroidism. Cohen and colleagues16 followed a cohort of patients who were treated with radiation to the tonsils before the age of 16 years. Among the 2923 patients, 32 were found to have clinical hyperparathyroidism—a 2.5- to 2.9-fold increase compared with the general population in the same age group. A long latency period (>25 years) occurs between exposure and the onset of hyperparathyroidism. Parathyroid adenomas were found in most of the patients in whom this complication develops.17 Clinical presentations vary from asymptomatic increases in serum parathormone levels and hypercalcemia to disabling metabolic bone disease or nephrolithiasis. Persons with a history of head and neck radiation should be monitored with calcium levels periodically (every 1 to 2 years) and indefinitely. Effects of systemic chemotherapy on ovarian and testicular function are discussed in Chapter 60. In children, chemotherapeutic agents without any cranial irradiation may disrupt growth hormone (GH) secretion. Roman and colleagues18 studied growth and GH secretion in 60 children who were in complete remission after treatment with chemotherapy and surgery for solid tumors. They observed GH deficiency in 45% of those studied and found that the GHD group had received significantly higher doses of actinomycin D compared with the non-GHD group (P < .05). These investigators found no correlation with duration of treatment, length of follow-up, tumor type, sex, or age. Depending on the intensity of chemotherapy, significant height loss was detected in 40% to 70% of patients at 6-year follow-up. Adjuvant chemotherapy can also aggravate growth failure in children with brain tumors who receive craniospinal radiation.19 Rose and colleagues20 reported hypothalamic dysfunction in patients with non–central nervous system tumors who received chemotherapy but did not receive cranial irradiation and had no history of traumatic brain injury. Of 31 identified patients, GHD was identified in 15 (48%), central hypothyroidism was identified in 16 (52%), and pubertal abnormalities was identified in 10 (32%). GHD and hypothyroidism were coexistent in eight patients (26%). Overall, 81% had GHD, hypothyroidism, precocious puberty, or gonadotropin deficiency. The syndrome of inappropriate antidiuretic hormone (SIADH) secretion is associated with various malignant tumors including certain primary brain tumors, hematologic malignancies, intrathoracic nonpulmonary cancers, skin tumors, gastrointestinal cancers, gynecologic cancer, breast and prostatic cancer, and sarcomas.21 SIADH may result from the effects of many chemotherapeutic agents, either by potentiation of antidiuretic hormone (ADH) effect or by increased ADH secretion. The most commonly implicated agents are vinca alkaloids and cyclophosphamide. The vinca alkaloids are reported to stimulate the central release of ADH from the neurohypophyseal system,22 whereas alkylating agents enhance renal tubular sensitivity to ADH.23 Regardless of the mechanism, the result is an increase in water reabsorption by the distal tubules of the kidney, leading to volume expansion and dilutional hyponatremia. Case reports also implicate platinum agents,24 vinorelbine,25 taxanes,26 and methotrexate.27 Clinically significant hyponatremia may occur with administration of these agents, and management requires fluid restriction and, at times, salt replacement. Clinically evident thyroid dysfunction has rarely been associated with the use of standard chemotherapy agents. However, a growing body of literature points to the increased prevalence of endocrine dysfunction after bone marrow transplantation, following use of preparation regimens that do not include radiation. Thyroid dysfunction has been reported in as many as 50% of allogeneic bone marrow transplant recipients treated with busulfan and cyclophosphamide.28 Thyroid dysfunction may appear as low T3 syndrome (i.e., normal levels of free T4 and TSH and a below-normal level of free T3), chronic thyroiditis, and transient subclinical hyperthyroidism or hypothyroidism. Chemotherapy may potentiate radiation-induced damage to normal tissue. Newer biological agents, tyrosine kinase inhibitors, and immune-based cancer treatments (e.g., ipilimumab, interferon-α, and interleukin-2) have been associated with various types of thyroid dysfunction.29, 30 Box 61-3 summarizes the well-described adverse effects of cancer therapies on thyroid function.
Endocrine Complications
Introduction
Role of Surgical Therapy
Role of Radiation Therapy
Hypothalamic-Pituitary Axis
Thyroid
Parathyroid Glands
Role of Systemic Therapy
Hypothalamic-Pituitary Axis
Thyroid
Endocrine Complications