and Winfried G. Rossmanith2
(1)
Lenzkirch, Germany
(2)
Ettlingen, Germany
10.1 Overview
10.2.1 The Hypothalamus
10.2.2 The Pituitary Stalk
10.2.3 The Pituitary
10.2.4 The Pineal Gland
10.3.1 The Thyroid Gland
10.3.2 The Parathyroid Glands
10.4 The Adrenal Glands
10.4.1 The Adrenal Cortex
10.4.2 The Adrenal Medulla
10.5.1 The Stomach
10.5.2 Duodenum and Ileum
10.8 The Gonads
10.8.1 Gonad Development
10.8.2 Male Gonads: Testes
10.8.3 Female Gonads: Ovaries
10.9.1 Neurosecretion in Mollusks
In this chapter, by presenting the anatomy of endocrine organs, we wish to demonstrate the differences between endocrine glands such as the adrenal glands and the thyroid gland and endocrine tissues of the gastrointestinal tract and neurosecretion in the brain.
10.1 Overview
The endocrine system appears to be hierarchically organized. Metabolism, growth, reaction to coldness, heat, or threat, and reproduction are all centrally controlled. It has not been know for long whether there is a master gland and where it is located. Many different organs, first of all the heart, were considered as controlling the inner functions of the physiology. Later, the pituitary was assumed to be the master gland since its hormones act in the thyroid gland, the adrenal glands, and the gonads. The perception of the superior function of the hypothalamus is a relatively recent development.
It is basic knowledge that all endocrine power emanates from the hypothalamus. Here the messages from the different organs are neuronally integrated. From the hypothalamus, releasing hormones are sent to the pituitary to release hormones controlling the thyroid gland, adrenal glands, and gonads. Many hormones of the gastrointestinal tract act like a type of reflex, bypassing the central control. These stimulate functional properties of other gastrointestinal tract cells directly. The organs of the gastrointestinal tract, however, are densely innervated, but the receptors of the endocrine signals from different hormone-producing cells in the gut and other organs of the digestive tract are in endocrine loops.
10.2 The Endocrine System of the Brain
At least three brain sections are part of the endocrine system: the master “gland” of the endocrine system—the hypothalamus—the pituitary or hypophysis, and the pineal gland. Whereas the hypothalamus and posterior pituitary are composed of neurons and neurosecretory cells, the anterior pituitary and the pineal gland are bona fide glands.
10.2.1 The Hypothalamus
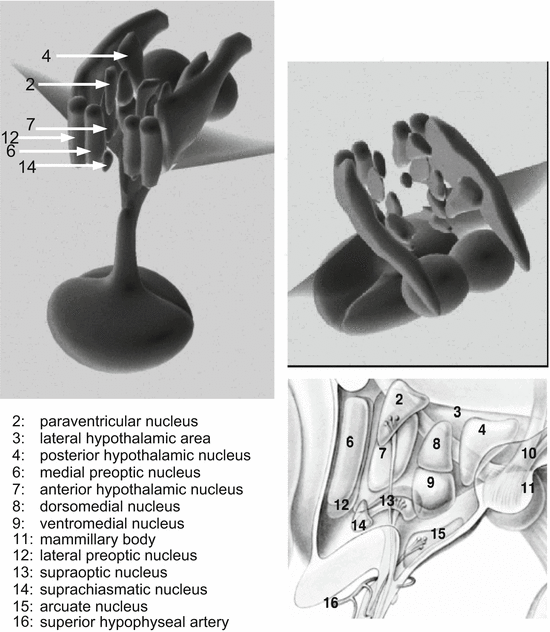
Those brain areas at the side or in front of the third ventricle are called the hypothalamus . The third ventricle is a fluid-filled space in the diencephalon .
Like other brain areas, the hypothalamus has distinct nuclei. These are centers with many neurosecretory cells of similar function. Whereas the nerve axons which, for example, in the case of the optic nerve extend from the eye to the brain or in the case of motor nerves extend from the brain to the peripheral muscles are usually covered by a sheath of connective tissue cells which form the stable blood–brain barrier, in the hypothalamus the nerves are unsheathed. This allows multiple connections between controlling nerve cells and hormone-producing neurosecretory cells and within neurosecretory cells. Nuclei in this context are not the cellular nuclei.
Different nuclei and areas of the hypothalamus have different functions in endocrine control (Fig. 10.1, Table 10.1).
Table 10.1
Hypothalamic nuclei
Region | Hormone synthesis and actions |
|---|---|
Arcuate nucleus | Growth-hormone-releasing hormone; gonadotropin-releasing hormone; glucagon-like peptide |
Dorsomedial nucleus | Orexin |
Paraventricular nucleus | Thyrotropin-releasing hormone; angiotensin II; somatostatin; vasoactive intestinal peptide; oxytocin; vasopressin; corticotropin-releasing hormone |
Preoptic nucleus | Gonadotropin-releasing hormone; lipopolysaccharide-induced fever (μ-opioid receptors) |
Suprachiasmatic nucleus | Vasoactive intestinal peptide; gonadotropin-releasing hormone; circadian pacemaker connected to the optic nerve, together with the preoptic nucleus involved in sleep regulation |
Supraoptic nucleus | Angiotensin II; oxytocin; vasopressinZ |
Ventromedial nucleus | Leu-enkephalin; substance P; neurotensin; glucose/insulin regulation; glucagon-like peptide; “threat, attack, flight” |
Anterior hypothalamic area | |
Posterior hypothalamic area | |
Dorsal hypothalamic area | |
Lateral hypothalamic area | Calcitonin-like peptide; 5α-testosterone reductase |
Neurosecretory cells of the hypothalamus differ from other nerve cells in that they do not control other cells via synapses; rather, they release their hormones into the circulation. For this purpose, the axons of these neurosecretory cells reach into the median eminence, an area at the bottom of the hypothalamus. Therein blood capillaries of a portal system have many small openings, so-called defenestrated areas or sieve plates . Once a neurosecretory cells has released its hormones in the median eminence, these hormones reach the circulation through the little windows. Although brain arteries are usually sheathed by the blood–brain barrier, blocking, for example, lymphocyte migration into the brain, in the median eminence and other neurohemal organ s—for example, in the posterior pituitary—the blood–brain barrier is nonexistent and the endocrine products reach the circulation directly. Neurohemal organs exist in mollusks as well in other non-vertebrates, and thus are not exclusively found in vertebrates.
It is an obvious question whether white blood cells, bacteria, or viruses can enter the brain via neurohemal organs—for example, tick-borne encephalitis virus. There is apparently no report of this in the literature. Most probably the openings are too small to allow white blood cells to enter. However, nasal sprays for administration of drugs which enter the brain along the olfactory nerve have been offered for a few years. The blood–brain barrier is equally permissive in the vascular organ of the lamina terminalis , in direct vicinity of the preoptic nucleus, as well as in the circumventricular organs (see Fig. 11.15); angiotensin II from the circulation, for example, can thus reach receptors on the surface of nerve cells. Via these connections, other hormones such as leptin and other neuropeptides can find their central receptors.
Neurosecretory cells of the hypothalamus are interconnected with other areas of the brain. Nerve cell axons reach certain hypothalamic nuclei and form synapses there.
10.2.2 The Pituitary Stalk
By the median eminence hormones are transported directly to the pituitary via a portal system. Special neurosecretory cells reach beyond the median eminence into the posterior pituitary, the neurohypophysis. These nerve cells and connective tissue form the pituitary stalk, the infundibulum. The blood vessels around the pituitary stalk transport the neuropeptide from the median eminence to the anterior pituitary, the pars distalis. Below the pituitary stalk there is the pituitary (see the description of calendar cells in Sect. 12.4).
10.2.3 The Pituitary
Below the hypothalamus in a hole in the sphenoid bone—the bone on which the brain lies—is the pituitary (Fig. 10.2). It is joined to the brain via the pituitary stalk and its surrounding blood vessels. The human pituitary is composed of the anterior pituitary and the posterior pituitary, aka the neurohypophysis (lobus nervosus). The human pars intermedia of the pituitary is only discernible during the fetal period and in children. In animals, the pars intermedia provides the hormones important for coloring and color adaption to the environment. Since we humans adapt no longer by hormonal activity and coloring but by behavior to our environment (a dark red face indicates not color adaption but a strong bloodstream in the facial capillaries), this part of the pituitary could degenerate.
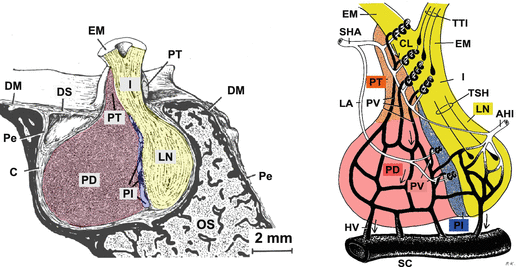

Fig. 10.2
The pituitary—an outline: general anatomy (left) and blood supply (right). In the sella turcica of the sphenoid bone (os sphenoidale, OS) the pituitary is placed with the adenohypophysis (anterior pituitary; pars distalis, PD), an intermediate region (pars intermedia, PI), and the neurohypophysis (posterior pituitary; lobus nervosus, LN). The capsule (C) of the pituitary is followed by the periosteum (Pe) of the sphenoid bone and its dura mater (DM). By the pituitary stalk (infundibulum, I), nerve axons from different hypothalamic nuclei reach the pituitary. Axons in the tuberoinfundibular tract (TTI) originate from neurosecretory cells of the dorsomedial nucleus, ventromedial nucleus, or arcuate nucleus ending mostly in the median eminence (eminentia mediana, EM); axons in the supraopticoparaventriculohypophysial tract (tractus supraopticoparaventriculohypophysialis, TSH) are from the preoptic nucleus or paraventricular nucleus and reach the neurohypophysis. The upper part of the anterior pituitary is called the pars tuberalis (PT). Using a portal system, hypothalamic neuropeptides from the median eminence get into the anterior pituitary. The superior hypophyseal artery (SHA) serves capillary loops (CL) of the median eminence and via the loral artery (LA) the capillary loops of the pars intermedia; the inferior hypophyseal artery (IHA) supplies blood to the neurohypophysis. Within the capillary loops, neuropeptides are taken up and reach the adenohypophysis via portal veins (PV ), and anterior pituitary hormones arrive via the portal veins in the cavernous sinus (SC) (Modified from Krstić (1991): left plate 122, right plate 128)
10.2.3.1 The Anterior Pituitary
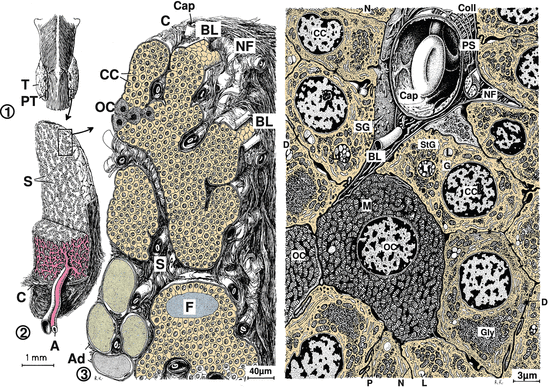
The anterior pituitary (aka the adenohypophysis or pars distalis) contains those cells which release adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone, growth hormone, and prolactin (Fig. 10.3) . In the thyroid gland and in the different zones of the adrenal glands, only one hormone is made. In the anterior pituitary as well as in pancreatic islets, adjacent cells release different hormones. (These hormones have already been discussed in Sect. 4.4).
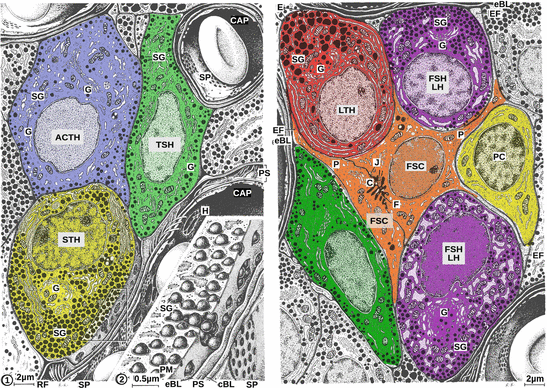

Fig. 10.3
Endocrine cells of the anterior pituitary. The following cell types can be distinguished: adrenocorticotropic cells (ACTH), somatotropic cells (STH), lactotropic cells (LTH), gonadotropic cells (FSH and LH), and thyrotropic cells (TSH). In addition there are follicular stellate cells (FSC) and precursor cells (PC) with no detectable hormone synthesis. All these hormone-synthesizing cells are characterized by a visible Golgi apparatus (G), numerous mitochondria, pronounced rough endoplasmic reticulum, and very strikingly, an enormous number of secretory vesicles (SG). On stimulation by hypothalamic releasing hormones, these vesicles fuse with the cell membrane, and release their content. Secreted hormones need only pass the short distance through through basal lamina and the perivascular space (PS) before reaching the capillaries via sieve plates (SP). Therein, capillaries are fenestrated to allow prompt uptake of hormones (left, magnification). Sieve plates are characteristic features of endocrine organs. C cilia, CAP capillary, cBL capillary basal lamina, E exocytosis, eBL external basal lamina, EF end feet, H holes, J junctions, P cytoplasmic processes, PM plasma membrane, RF reticular fibers (Modified from Krstić (1991), plates 124 and 125)
Adrenocorticotropic Cells
These are polygonal cells with an eccentric cellular nucleus; secretory granules are mostly found at the cell margins. These cells can be stained by neither basophilic nor acidophilic dyes.
Secretion of ACTH is controlled by corticotropin-releasing hormone .
Somatotropic Cells
These are rounded cells of medium size with a globular cellular nucleus and a conspicuous nucleolus, large electron-dense secretory granules1 distributed all over the cytosol, large rough endoplamic reticulum cisternae, and well-developed mitochondria ; these cells can be stained by acidophilic dyes.
Growth hormone secretion is controlled by growth-hormone-releasing hormone and somatostatin.
Thyrotropic Cells
These are irregularly shaped, polygonal, elongate or stellate cells with an elliptic cellular nucleus and well-developed organelles; osmiophilic2 secretory granules are grouped in the cell periphery; granules can be stained by basophilic dyes.
Release of thyroid-stimulating hormone is under the control of hypothalamic thyrotropin-releasing hormone .
Lactotropic Cells
These are rounded cells with an oval cellular nucleus; mainly during pregnancy and lactation there are many large secretory granules . These granules are acidophilic .
Secretion of prolactin is steadily inhibited by dopamine . A functional hypothalamic prolactin-releasing hormone has not yet been discovered.
Gonadotropic Cells
These are oval cells with a rounded cellular nucleus. Osmiophilic secretory granules arise from the Golgi apparatus. The cells are basophilic.
Secretory granules contain simultaneously FSH and LH , whose release is controlled in humans by hypothalamic gonanotropin-releasing hormone type I. An individual FSH-releasing hormone has been postulated anecdotally, but the fact that both hormones are found in the same vesicles argues against a special FSH-releasing hormone.
10.2.3.2 The Posterior Pituitary
In contrast to the anterior pituitary, in the posterior pituitary (Fig. 10.4) there is no hormone synthesis, only release. In the posterior pituitary, the axons of neurosecretory cells from the paraventricular nucleus, ventromedial nucleus, supraoptic nucleus, and arcuate nucleus end at capillaries to release their hormones into the pituitary portal system (Fig. 10.4).
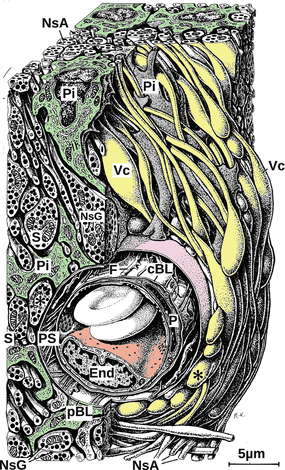

Fig. 10.4
Section of the posterior pituitary. Axons of arginine vasopressin and oxytocin neurons release vasopressin and oxytocin. Neurosecretory axons (NsA) end at capillaries. Neurosecretory axons are full of neurosecretory granules (NsG). Between the neurosecretory axons there are pituicytes (Pi), irregularly shaped stellate cells, whose endings are in intimate contact with neurosecretory axons. Hormones when released reach the capillaries and thus the circulation by passing first the pericapillary basal lamina (pBL), then the pericapillary space (PS), and finally the fenestrated capillary basal lamina (cBL). End endothelial cell, F fibrocytic process, P pericyte, S synaptic vesicle, Vc varix, asterisk enlarged axon endings (Modified from Krstić (1991), plate 127)
The posterior pituitary is like the median eminence, a neurohemal organ where brain cells release their content into the circulation and where the blood–brain barrier is permissive, where capillaries have fenestrated sieve plates for taking up released hormones. The two type of neurosecretory cells there release two hormones with closely related structures but different functions: oxytocin and vasopressin.
10.2.3.3 The Pars Intermedia
In the intermediate region or pars intermedia located between the anterior and the posterior pituitary, proopiomelanocortin is cleaved to melanocyte-stimulating hormone (MSH ) instead of ACTH as in the anterior pituitary. MSH controls coloring in amphibians, where the pars intermedia is markedly developed. MSH in humans regulates appetite and hunger, but it is not made in the pars intermedia.
10.2.4 The Pineal Gland
The pineal gland (Fig. 10.5) got its name (glandula pinealis) from its cone-like form and not because of its acervuli , crystalline calcium phosphate that looks like fir cones or raspberries. No function has ever been attributed to these acervuli; the age-dependent buildup of calcium-enriched and calcium-deficient layers points to seasonally different deposition of calcium ions. The characteristic cell of the pineal gland is the pinealocyte, which synthesizes and releases melatonin in dark—light cycles (see Sect. 7.3).
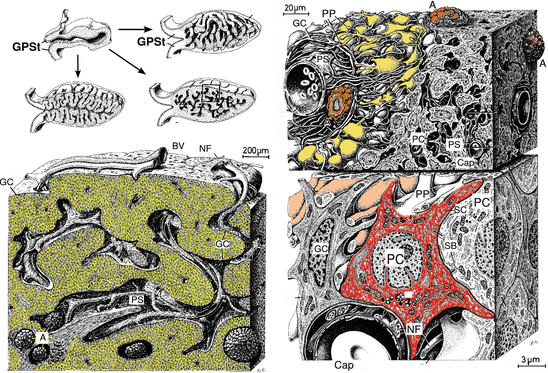

Fig. 10.5
The pineal gland. The pineal gland (glandula pinealis, epiphysis cerebri) got its name from its cone-like shape. Upper left: The anastomizing cords of the pineal gland develop from a diverticulum of the diencephalon by branching or crooking. Septa reach from the capsule into the inner pineal gland. Glial cells (GC) separate glandular tissue and septa, however in an incomplete flat layer. Lower left: Within spaces there are arteries, veins (BV ), and nerves (NF). Within the perivascular and pericapillary spaces as well as in the parenchyma there are (visible by X-ray) acervuli (A) made of calcium apatite and calcium carbonate. Upper right: Pinealocytes secrete hormones via processes (PP) near capillaries. Lower right: The typical pineal cell is the pinealocyte (PC), which releases melatonin. The pinealocyte cellular nucleus is partially deeply invaginated. Smooth endoplasmic reticulum close to the membrane forms characteristic cisternae (SC). Synaptic bars (SB) occur close to the plasma membrane. Cap capillary, GPSt pineal stalk, PS perivascular space (Modified from Krstić 1991, plates 129 and 130)
Innervation of the pineal gland occurs via nerve fibers through the pineal stalk and via septa. Adrenergic fibers of the coronary nerve connect the superior cervical ganglion (not covered by myelin sheaths) with the pineal gland. These fibers do not cross the pineal stalk. Axons reaching the pineal gland via the pineal stalk originate in the posterior commissure and the thalamus. In addition, the pineal gland receives signals from the retina by which the light-inhibited melatonin synthesis is controlled.
The pineal gland of amphibians reacts to light. In humans there are neuronal connections to the optic nerve, which suggests some sensing of light.
Melatonin is the characteristic hormone of the pineal gland. Its biosynthetic intermediate serotonin is a important neurotransmitter in the central nervous system. Melatonin biosynthesis is controlled by the suprachiasmatic nucleus, which triggers, together with noradrenergic nerves, in the absence of light transcription of arylalkylamine N-acetyltransferase (see Sect. 7.3).
10.3 The Thyroid and Parathyroid Glands
10.3.1 The Thyroid Gland
The thyroid gland (Fig. 10.6) is situated below the larynx in front of the trachea. The gland is covered by a thick capsule of connective tissue. Such connective tissue also separates the characteristic follicles from each other. Follicles are formed by a unicellular layer of follicular cells surrounding an amorphic gelatinous colloid .
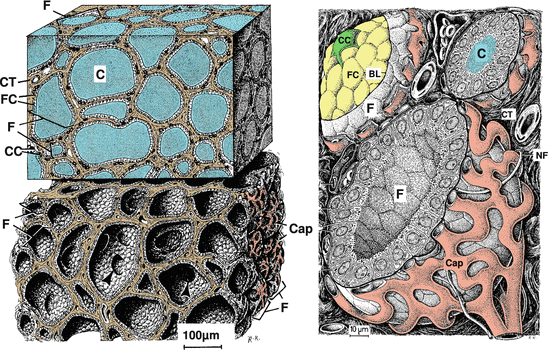

Fig. 10.6
The thyroid gland. Upper left: Section of the thyroid gland, follicles filled with colloid. Lower left: Similar section, follicles empty. Right: Individual follicles are magnified. Characteristic of the thyroid gland are the follicles (F) filled with colloid (C) which are lined by a unicellular follicular cell (FC) layer. Around the follicles there is a basal lamina (BL), connective tissue (CT), and a dense network of fenestrated capillaries (Cap). On the fenestrated capillaries there are some C cells (CC), which are darkened in the upper-left image. From the capillaries, follicular cells take up iodine, which they couple to thyroglobulin. Such iodothyroglobulin is stored in the colloid. Stimulated by thyroid-stimulating hormone, follicular cells pinocytose lipid droplets, cleave thyroxine from thyroglobulin, and release this thyroxine. C cells generate and secrete calcitonin. NF nerve fiber (Modified from Krstić (1991), plates 131 and 132)
Follicular cells generate triiodothyronine and thyroxine—hormones involved in metabolic homeostasis. Iodide actively taken up by follicular cells is oxidized by thyroid peroxidase to iodine, which is then used to derivatize tyrosine residues of thyroglobulin. Iodinated thyroglobulin is stored in the colloid. Triggered by thyriod-stimulating hormone, iodothyroglobulin is taken up by endocytosis into vesicles called phagosomes . Phagosomes fuse with lysosomes to form phagolysosomes, where thyroxine is enzymatically removed from thyroglobulin. Thyroxine and eventually derived triiodothyronine diffuse into the capillaries surrounding the follicles.
There is another cell type in the thyroid gland, so-called C cells. These cells have osmiophilic granules where calcitonin and somatostatin are generated and stored.
10.3.2 The Parathyroid Glands
On the back of the human thyroid gland, there are four small glands, the parathyroid glands (Fig. 10.7). In reptiles, there are additional parathyroid glands found in pairs along the brain artery. Below a capsule of dense connective tissue, the glandular parenchyma is invaginated by connective-tissue-lined septa containing capillaries. From the stroma, the parenchyma is separated by a visible basal lamina.