The Emergence of New Inflammatory Markers of Head and Neck Cancer and their Potentials
Norhafiza Mat Lazim1, *, Baharudin Abdullah1
Abstract
Inflammation plays a critical role in the process of carcinogenesis as well as in modulating treatment effects of many therapeutic agents for head and neck malignancy. Current research indicates that a myriad of new diagnostic tools and treatment modality works in harmony in producing a desired effective cancer treatment regime. Imperatively, multiple inflammatory markers have surged in the genomic and molecular ecospheres as highly potential agents that can be used in screening, diagnosis, treatment as well as follow up of head and neck cancer patients. These markers include arrays of cytokines, peptides, macrophages, acute phase proteins, growth factors, and many more. The tumor microenvironment is a complex ecosystem and has an intricate relationship with its surrounding biosphere. The multiple interactions within the molecules in the cancer microenvironment play significant roles in mediating and promoting carcinogenesis as well as mitigating the treatment response. These complex ecosystems are also responsible for the occurrence of metastatic diseases, recurrences, and residual diseases. This chapter highlights some of the critical inflammatory markers that can be potentially used as a potent theranostic approach for head and neck tumors in the near future.
* Corresponding author Norhafiza Mat Lazim: Department of Otorhinolaryngology-Head and Neck Surgery, School of Medical Sciences, Universiti Sains Malaysia, Health Campus 16150, Kubang Kerian, Kelantan, Malaysia; Tel: +60199442664; E-mail: norhafiza@usm.my
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is known to be aggressive, with a high incidence of local recurrence and distant metastases despite multimodality treatment. This may be attributed to the heterogeneity of the populated tumor cells as wells as tumor and patient’s biology. Patients who are exposed to inflammatory agents such as alcohol, smoke, viruses, environmental
chemical agents, and selected dietary factors are at greater risk of developing HNSCC. Selective groups of HNSCC are resistant to treatment and prone to develop metastatic disease. They are known to have aggressive natures and a tendency to recur. Clinical presentation of each type of HNSCC varies and depends on the primary anatomic locations and organ involvement. The majority of the patients are in the middle and older age group, with a smaller percentage of the pediatric patient population, who are at increased risk of developing HNSCC. Assessment of HNSCC requires a multi-step process and numerous procedures, and the involvement of a dedicated team is a pre-requisite for optimal treatment planning and execution.
Importantly, the risk factors of HNSCC are strongly related to inflammation. For instance, viruses, dietary factors, and environmental carcinogens always cause acute inflammation as an initial insult, which then progresses to chronic inflammation. This later will be followed by the development and promotion of carcinogenesis. Multiple factors and molecules are involved in the process of carcinogenesis originating from the initial inflammation insult. The interaction between these factors in the tumor microenvironment is responsible for the progression of carcinoma. Several excellent reviews have discussed the probable role of cell and molecular inflammation in cancer growth. Most studies reported consistent associations between chronic inflammatory conditions and inflammatory-inducing risk factors, such as alcohol and tobacco consumption, the oncogenic viruses (EBV and HPV), wood dust exposure, in carcinogenesis of head and neck malignancy [1–3].
The process of carcinogenesis is complex and involves multifactorial tissue elements. It is an intricate process, which commonly involves a primary insult, mitigated by factors that can further promote carcinoma development. Peyton Rous was the first to identify that cancers arise from “neoplastic substrate states” induced by viral or chemical carcinogens that trigger somatic changes. These somatic changes are critical insult points in cancer progression. They can be induced by carcinogenic stimuli like chemical carcinogens, nitrosamines in the preserved vegetables and salted fish, acetaldehyde from alcohol, and many chemical components in tobacco, aflatoxins, aromatic amines, and other genotoxic compounds [4]. The steps of initiation and promotion are crucial in carcinogenesis and are both modulated by multiple factors. Significant DNA changes occur during the initial stage, and they are mostly permanent. Chronic exposure to inflammation-induced factors serves as the second stage i.e. the promotion. This multistep process of carcinogenesis is very complex and requires intricate genomic changes and chronic exposure of the mucosa of head and neck cancer subsites [5]. It is crucial to understand the true process of carcinogenesis involving the inciting agents that illicit inflammatory cascades.
Other tissue factors are also equally crucial in the development of carcinoma and mitigating carcinogenesis. This can involve vascularization, genetic aberrations, and other deranged physiological processes in a specific microenvironment. Indeed, inflammation is a mechanism that facilitates many forms of cancer. It is suspected to influence the development and progression of cancer through many etiological pathways. These include increased levels of DNA adduction, mutations of oncogenes and tumor suppressor genes, increased vascularization, aberrant anti-apoptotic signaling, and immune evasion [1, 6, 7]. DNA methylation especially hypermethylation, has been known to cause certain HNSCC such as oral cavity carcinoma and salivary glands carcinomas. The process of angiogenesis is paramount as most of the tumoral mass displays various patterns of vascularization. This angiogenic pattern is responsible for tumor characteristics and the true biology of the HNSCC. Several of these processes may interact with each other in producing harmful effects that lead to carcinogenesis and malignant mass formation.
INFLAMMATION AS A PRIMARY CAUSE OF CANCER PROGRESSION
Although cancer is multifactorial in origin, various epidemiological and experimental studies suggest that chronic inflammation has an important role in all stages of cancer, from initiation to progression and even survival of the patient. Inflammatory products like cytokines, chemokines, leucocytes, prostaglandins, cyclooxygenase, reactive oxygen, and nitrogen species are all involved in the development of carcinomas. The other equally important elements are the aberrant genetic factors and processes such as metalloproteinase that able to induce genetic and epigenetic changes in normal cells and damaging their DNA. They can also inhibit their repair, modify transcription factors, suppress apoptosis, and induce angiogenesis, resulting in carcinogenesis. All these markers play a vital role not only in promoting cancers but also in diagnosis, assessment, treatment, and post-treatment surveillance. Thus, these inflammatory mediators have a potential role to become cancer biomarkers for all stages of cancer as many of them can be measured in a cost-effective manner [8]. These biomarkers may emerge as potential therapeutic agents in combatting head and neck malignancy from the very beginning.
The role of inflammation in cancer is indisputable. To illustrate this strong relationship further, the recent literature has highlighted many chronic inflammation reactions as the primary causes of the majority of solid and epithelial malignancies in humankind globally. Numerous writers have reported that many studies highlight the strong association of inflammation with chronic diseases. Accumulating data indicates that chronic inflammation is a precursor to tumor formation such as gastric carcinoma, hepatocellular carcinoma, colonic carcinoma, cervical cancer, and so forth [9–11]. Myriads of molecules and markers have been identified that able to promote cascades of an event, which eventually lead to cancer development. Gastritis i.e., inflammation of the lining of the stomach, is the initial insult that eventually leads to gastric cancer. The persistence of chronic active infection in gastric mucosa, together with the presence of H. pylori infection, stimulates the initial phase of invasive gastric carcinoma [10]. Lung cancer has been strongly related to cigarette smokers, which results in numerous molecular changes and inflammation that lead to malignancy [12]. Similarly, patient with inflammatory bowel disease has a higher incidence of colorectal carcinoma [13, 14]. Indeed, this cancer-related chronic inflammation plays critical key roles in various aspects of tumor growth, including the proliferation of malignant cells, cell transformation and survival, the progression of invasion, systemic metastases, and tumor response to the drugs.
Increased development of pro-inflammatory mediators, such as cytokines, chemokines, and reactive oxygen intermediates, are the molecular mechanisms by which chronic inflammation drives cancer initiation and promotion. Other factors and components, such as increased oncogene expression, cyclo-oxygenases, matrix metalloproteinase (MMPs), and pro-inflammatory transcription factors, are responsible for tumor progression and response to treatment [15]. These inflammatory-associated molecules are activated by a variety of environmental and carcinogenic stimuli, including the virus, tobacco and alcohol consumption, chemical reagents, and so forth. Of note, all of these in combination are thought to drive the majority of human solid malignancies.
Although inflammation promotes the development of cancer, microenvironmental tumor components such as tumor and stromal cell, inflammatory and immune cells infiltrate produce many stimulative pro-inflammatory molecules that play important roles in tumor initiation, promotion, and progression [16]. Recent studies have shown that cancer-related inflammation results from contact between the host and tumor cells to create a reciprocal interplay that often leads to structural changes, immune suppression, evasion, and malignant progression [17]. Overexpression and abnormal activation of these pro-inflammatory mediators further promote tumor promotion and progression. It is this complex interaction that exists within a tissue biosphere that needs to be comprehensively studied by scientists and clinicians in order to discover novel markers.
Additionally, Tumor-infiltrating lymphocytes (TILs) may also be involved in carcinogenesis. TILs are thought to function in both promoting tumor growth and changes of treatment response to for instance chemotherapy due to its anti-tumor immunity effects. The lymphocytes count in relation to the neutrophil count has also been investigated and shoed to have prognostic significance [18]. This inflammatory marker, neutrophil-lymphocyte ratio (NLR) has been reported to have a strong association with recurrence and survival in HPV-positive cancers [19–21]. Importantly, organ transplantation often raises both local and systemic levels of inflammation and is associated with elevated rates of malignancies. Inflammatory markers, such as interleukin-6 (IL-6), tumor necrosis factor and C-reactive protein (CRP), have been shown to increase tissue damage and active disease and influence surgical outcomes [22, 23]. Both cancer risk and plasma levels of inflammatory markers increase with age. Importantly, the levels of circulating inflammatory markers are associated with an increased risk of cancer [22]. This critical finding mandates further intense research as it has a significant impact on the armamentarium of head and neck oncology management.
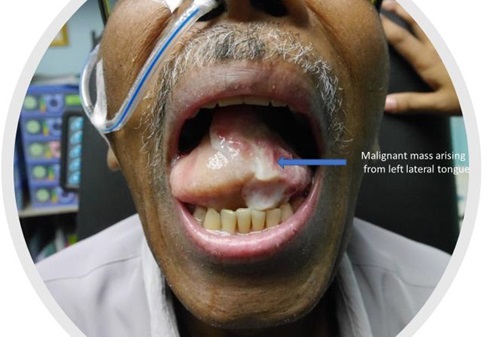
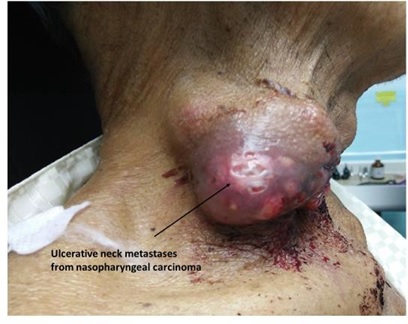
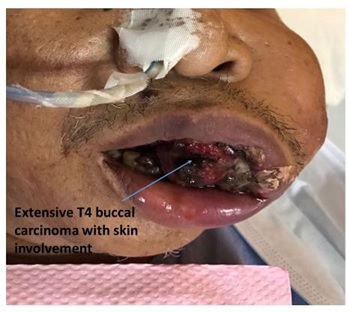
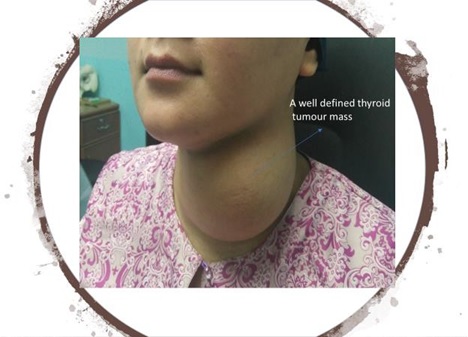
Some of the head and neck malignancies that are highly related to inflammation include oral cavity carcinoma, nasopharyngeal carcinoma, buccal carcinoma, and thyroid tumors (Figs. 1–4).
Fig. (1))
Carcinoma of left lateral border of the tongue.
Fig. (2))
Nasopharyngeal carcinoma patient with neck node metastases. The malignant tumor has breached the skin.
Fig. (3))
Oral cavity carcinoma ie extensive T4 buccal carcinoma.
Fig. (4))
Multinodular goiter in a young lady.
EPIDEMIOLOGY OF HEAD AND NECK TUMOUR AND INFLAMMATION DRIVEN CAUSES
According to the WHO cancer statistics, the incidence of head and neck cancer showed an increase in trends, in the majority of the continents especially in the area where the living status is suboptimal. At certain geographic locations, more patients have been diagnosed annually with head and neck cancers includes pediatric malignancies. There have been many identifiable risk factors that have been and are currently being investigated and linked to the carcinogenesis of head and neck malignancy. Oral cavity carcinoma is highly prevalent in India and is strongly related to betel nut chewing and reversed smoking. On the other hand, nasopharyngeal carcinoma is known to be prominent in the South East Asia region, china, Taiwan, Hong Kong, and Japan. This different geographic location reflects different inflammatory agents that may have involved in this malignancy.
Chronic diseases, obesity, alcohol, tobacco, radiation, environmental contaminants, and high-calorie diets have been reported as significant risk factors for the most common forms of cancer including head and neck malignancy [24, 25]. Most of these risk factors are related to cancer progression via inflammation. Imperatively, acute inflammation mediates host protection against infections, long-term chronic inflammation can predispose the host to various chronic diseases, including cancers [26]. In the head and neck region, chronic infection with EBV is well known with nasopharyngeal carcinoma whereas chronic infection of human papilloma virus is associated with laryngeal and oropharyngeal tumors [27]. Smoke inhalants, wood dust, smoked seafood, salty fish, and pickled vegetables can all induced malignancy like sinonasal carcinoma and NPC through the chronic inflammation process. The many interactions that exist between the molecules and tissue vasculature in the specific tissue microenvironment driven a lethal pathogenic cascade that eventually leads to the formation of a tumor.
It is crucial to understand the process of inflammation. Inflammation can be divided into two stages, acute and chronic inflammation. Acute inflammation is a part of the innate immunity of immune cells, which lasts for a short period. There are multiple markers involved in this phase that primarily act to initiate tissue healing and repair. However, if the inflammation continues, the second stage of inflammation called chronic inflammation persists. This chronic inflammation underlies the pathogenesis of multiple chronic diseases and cancer via alteration of various signaling pathways and inflammatory cascades [28, 29]. Chronic inflammation triggers multiple critical events and produces changes that take place in the tissue with subsequent recruitment of particles and molecules which causes angiogenesis, granulation tissues, and scarring. Angiogenesis is an important element in tumor survivals. It also plays role in diagnostic as well as therapeutic for head and neck malignancies.
INFLAMMATION AS A POTENT RISK FACTOR FOR HEAD AND NECK MALIGNANCY
Clinical and epidemiological studies have shown a significant correlation between chronic infection, inflammation, and cancer. This correlation initially proposed by Rudolf Virchow when he observed an increased number of leukocytes were present in tumor tissues and indicated that tumors could be related to chronic inflammation [30]. Thereafter, a surge of literature focusing on inflammation and cancer took place and extensive research also starts expanding globally. Scientists, physicians, clinicians, epidemiologist, pathologist embarks on mutual collaborative works and studies in gathering information in order to get the data on the nature of solid malignancies in humans.
Numerous molecules that play a critical role in inflammation have been identified in the last two decades of intensive research. These include tumor necrosis factor (TNF), interleukin-chemokines, cyclooxygenases, matrix metalloproteases (MMP), a vascular endothelial growth factor that play major roles in carcinogenesis either in tumor progression or mediate treatment effects [26, 31–35]. As aforementioned, malignancy of the head and neck is associated with multiple environmental factors, dietary habits, smoking and alcohol, workplace hazards, chemical, and radiation exposures as well as viruses. Chronic inflammation due to these inciting agents is thought to encourage carcinogenesis and can predispose individuals to particular types of malignancy. Non-infectious chronic inflammation is also associated with several types of cancers including head and neck carcinoma [22]. This portrays numerous factors in the tissue ecosystem that trigger the inflammation cascade responsible for cancer development.
Thus far, we have seen that cancer is one of the main diseases caused by chronic inflammation. In 2009, Colotta et al. suggested cancer-related inflammation as the seventh mark of cancer [36]. This is a pertinent discovery that alters the focus of the study in the pathogenesis of head and neck malignancy. Both the internal and external pathways link cancer and inflammation. The oncogenes control the inflammatory microenvironment, while the inflammatory microenvironment facilitates the progression of cancer [28, 37]. The tumor microenvironment comprises a milieu of factors, protease, extracellular matrix, and proteins that are involved in a complex ecosystem to maintain and generate cancer stem cells [37, 38]. Many of these tumor microenvironment constituents play significant roles in head and neck malignancy, for instance in oral squamous cell carcinoma [39]. These carcinogenic stimuli and inflammatory inciting agents influence the level and the mode of actions of the TME elements in the carcinogenesis. Inflammatory stimuli may include bacterial infection H. pylori in gastric cancer and tobacco in lung cancer [26]. During the initiation stage, the addition of genetic changes is necessary for tumor formation to occur and progress. The carcinogenic stimuli initiate the mucosa changes but only with persistent mutational changes that will allow carcinogenesis to develop.
Promotion and progression of tumors rely on signals that derive from non-mutant cells in the microenvironment of the tumor. The microenvironment of the tumor consists not only of tumor cells but also of stromal cells, cells of the innate immune system, neutrophils, mast cells, myeloid-derived suppressor cells, macrophages, dendritic cells, lymphocytes, and so forth. This microenvironment can either facilitate tumor progression or become a potential barrier for tumor proliferation through a complex system of immune system interaction, angiogenic proliferation, and growth factors stimulations [40]. It can also be used in the development of anti-cancer therapy [41]. These cell types secrete cytokines, growth factors, proteases, and many more factors that can act in an autocrine or paracrine manner. During the carcinogenesis process, dampening effects of anti-tumor activity take place, and activated proinflammatory events and cascade occurs that lead to the formation of tumor, vascularization, and metastases [30].
Infectious organisms cause inflammation by activating receptors cell and interfering with the composition of wall components and nucleic acids. The mucosal breach facilitates the entry of the organism. Sometimes the architecture of the mucosa itself, like the tonsillar region which has multiple crypts is an ideal place for an oncogenic particle like the viruses to reside. The HPV positive oropharyngeal carcinoma is exclusively in reference to the tonsillar carcinoma, which has different presentations, treatment approach, and prognostication [42–44].
However, insufficient eradication of pathogens or persistent infection with prolonged inflammatory signaling and malfunctions in anti-inflammatory pathways and cascades can all contribute to chronic inflammation with the eventual development of malignancy [45]. Arrays of chemical carcinogens, nitrosamines, aflatoxins, tobacco, and alcohol also incite similar inflammation trends. By understanding this complex interaction and pathogenesis that exist between the carcinogenic stimuli and inflammatory changes, it will lead to new insights into cancer and inflammatory diseases and transcend common perspectives on cancer and inflammation [46, 47]. Tumor-associated macrophages and their mediators influence key elements in the multi-step phase of invasion and metastases [48]. Some reports stated that a higher number of TAM is associated with poor prognosis for example in oral cavity cancer and nasopharyngeal carcinoma [49–52]. TAM also has cute roles in angiogenesis, invasion, immune evasion, and tumor metastatic potential [53, 54].
Important activation of inflammatory mediators like interleukin and signaling pathways in the buccal epithelium was observed in subjects who burned smoky coal relative to smokeless coal [55]. TAM, lymphocytes, c-reactive proteins, and cyclo-oxygenase also have critical roles in the promotion of oral tumor carcinogenesis [56]. These inflammatory mediators also are a potential marker for the diagnosis and prognosis of oral cavity carcinoma. In order to identify the malignant transformation of neoplasms and to expose the functional mechanism that allows cancer cells to progress, the detailed cancer characteristics of cancer cells and their microenvironment need to be intensely investigated [46].
SIGNIFICANT INFLAMMATORY MARKERS IN HEAD AND NECK CANCER
There are six groups of an inflammation-related biomarker that have been identified which includes cytokines or chemokines, immune-related factors, acute phase proteins, reactive oxygen and nitrogen species, prostaglandins and cyclooxygenase-related factors, and mediators such as transcription and growth factors [1]. These biomarkers have numerous significant roles in carcinogenesis and malignancy development. Other markers that are associated with immune evasion have also been identified and are responsible for the immune escape mechanism for cell proliferation, invasion, and metastasize. These markers include macrophages, NLR, cytokines, growth factors, and TAMs.
Some of these markers can be used to give a clinical prediction of tumor behavior, response to treatment, and development of recurrence. Certain markers have been used to assess the risk of lymph node metastases in papillary thyroid carcinoma. Selected cytokines have been associated with the growth and development of human gastric carcinoma, colorectal cancer, and esophageal cancer [29]. Studies are also looking at the roles of cytokines in the prediction of head and neck cancer regional spread, for instance, cytokines produce due to radiation therapy [57, 58]. Cytokines that are present in saliva have been reported to be different between pre- and post-treatment which can be used to monitor treatment response [59, 60].
Clinical trials evaluating systemic inflammatory response in cancer patients for prognostication is escalating. The neutrophil-to-lymphocyte ratio (NLR) is one of the main biomarkers of systemic inflammation and has been associated with increased tumor burden and disease spread as well as monitor treatment response to immune-targeted therapy [61–63]. NLR is elevated in patients with laryngeal squamous cell carcinoma relative to those with benign and precancerous lesions. NLR is also an independent indicator of decreased overall survival in most epithelial cancers [64].
Tumor Microenvironment and Inflammatory Markers
In normal tissue, the cellular microenvironment is capable of suppressing malignant cell development. However, tumor-stromal interactions that modulate the microenvironment are more permissive to malignant cell proliferation and increase cell motility and adherence. This tumorigenic process includes angiogenesis, lymphangiogenesis, and multiple inflammation cascades that able to be amplified with the presence of growth factors, cytokines, proteases, and so forth. In addition, the composition and function of the basement membrane are generally altered in cancer, along with changes in growth factor expression, recruitment of inflammatory cytokines, and increased fibroblast proliferation, all of which lead to the metastatic spread of tumor cells. It is indeed to manage head and neck malignancy as in the majority of cases, treatment response is poor due to aggressive tumor and issues of chemo-radioresistance and cancer-prone to recurs. This will impair the patient’s survival and quality of life [65].
Of note, the tumor microenvironment is capable of influencing malignant cell growth by releasing ECM proteins, growth factors, and cytokine. Critically, tumors themselves secrete growth factors and proteases that are capable of altering their local microenvironment, promoting the interaction among the tumor cell themselves, and facilitate immune surveillance [66, 67]. This is a crucial relationship in the carcinogenesis events as these markers become disproportionately unbalanced, altering the cellular process within the ecosystem. Most current data support the notion that acute inflammation caused by tumor-infiltration of host leukocytes does not have natural immune-protective mechanisms that contribute to the eradication of cancer and antitumor immunity. Instead, overly and excessively developed pro-inflammatory mediators are thought to contribute to tumor promotion and progression.
In the microenvironment of the tumor, there is a delicate balance between antitumor immunity and tumor-originated proinflammatory activity, which weakens antitumor immunity that will allow the tumor to expand rapidly [45]. A summary of some of the critical markers is listed in Table 1.
| Groups of Inflammatory Biomarkers | Cancer Sites | Roles of Specific Markers |
|---|---|---|
| Peptide | HNSCC (Wright et al., 2016) [31] HNSCC (Yoshitake et al., 2015) [32] | • Tumor targeting peptides for to improve imaging sensitivity (angiogenic cyclic RGD peptide cilengitide) • 12 amino acid peptide HN1 may have relation to HPV positive HNSCC • Cancer vaccine therapy (Multiple tumor antigens TAA derived peptide) |
| Chemokines | Adenoid Cystic carcinoma of Salivary gland (Muller et al., 2016) [33] HNSCC (Muller et al., 2016) HNSCC (Wolff et al., 2011) [34] HNSCC (Antonio LP et al., 2015) [35] | • Chemokine receptors as indicative of metastases. (CXCR4) • May suppress apoptosis induced by chemotherapy agent of tumor load • Lymph nodes metastases (CCR7) • B Cell Lymphoma (CXCR5) • Chemotherapy Resistance • High CXCR4 expression increased risk of nodal invasion (high CXCR4 expression) • Low risk of local and distant recurrence (low CXCL12 expression) |
| Macrophages | OCSCC (Evrard et al., 2019) [36] HNSCC + OCSCC (Kumar et al., 2019) [37] | • TAMS indicate poor prognosis • correlate with increased lymph node metastasis, extracapsular extension, and advanced stage |
| CRP proteins | OCSCC (Katano et al.,2017; Chen IH et al., 2014, Chen HH et al., 2013) [22, 23, 38] | • Prognostic Markers • Lymph node metastasis • Recurrence • Clinical tumor status • Extracapsular (ECS) lymph nodes spread |
| Interleukins | HNSCC (Choudhary et al., 2016) [25] HNSCC (Aderhold et al., 2014) [39] HNSCC (von Biberstein et al., 1996) [40] | • Tumor stage (IL6 elevation • indicate high tumor stage • Lymph nodes positivity • Chemoradiotherapy resistance • Tumor Metastases and progression • IL 4 as a potential screening marker • unrestricted growth and metastasis of (Increased IL-1 index) |
| VEGF | HNSCC (Aderhold et al., 2014) [39] | • Tumor node metastases TNM staging |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree