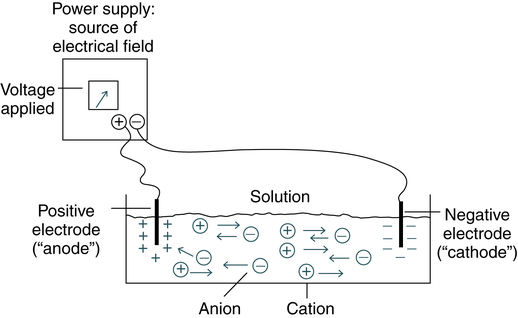
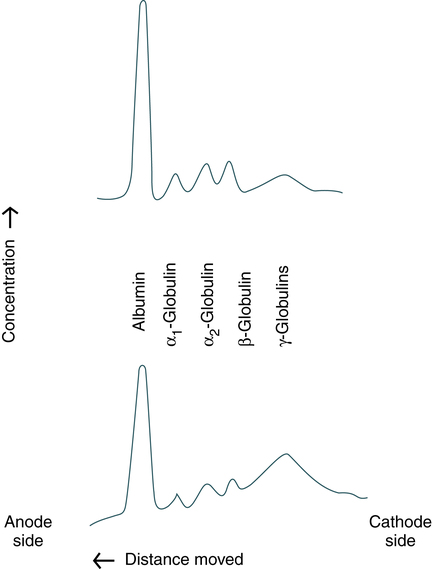
At the conclusion of this chapter, the reader should be able to: • Define the term electrophoresis. • Describe the electrophoresis technique. • Identify the fractions into which serum proteins can be divided by electrophoresis. • Describe the characteristics of immunoelectrophoresis. • Explain the features of immunofixation electrophoresis. • Discuss the clinical applications of immunoelectrophoresis. • Compare immunoelectrophoresis and immunofixation electrophoresis. • Compare capillary electrophoresis and microchip capillary electrophoresis. • Describe two electrophoresis separation methods. • Analyze a case study related to the results of serum protein electrophoresis. • Correctly answer case study related multiple choice questions. • Be prepared to participate in a discussion of critical thinking questions. • Describe the principle of the immunofixation electrophoresis procedure. The electrical field is applied to a solution with oppositely charged electrodes. Charged particles in this solution begin to migrate. Positively charged particles (cations) move to the negatively charged (−) electrode; negatively charged particles (anions) migrate to the positively charged (+) electrode (Fig. 11-1). Serum proteins are often separated by electrophoresis. Serum electrophoresis results in the separation of proteins into five fractions using cellulose acetate as a support medium (Fig. 11-2). This separation is based on the rate of migration of these individual components in an electrical field. Electrophoresis is a versatile analytic technique. Immunoglobulins are separated by electrophoresis using agarose as a support medium. The immunologic applications of electrophoresis include identification of monoclonal proteins in serum or urine, immunoelectrophoresis, and various blotting techniques (see Chapter 14). Immunodiffusion is a laboratory method for the quantitative study of antibodies (e.g., radial immunodiffusion [RID]) and rocket electrophoresis or for identifying antigens (e.g., Ouchterlony technique). Single diffusion preceded radial immunodiffusion. In the single diffusion procedure, antigen was layered on top of a gel medium and, as the antigen moved down into the gel, precipitation occurred and migrated down a tube in proportion to the amount of antigen present. In radial immunodiffusion (RID) (Fig. 11-3), antibody is uniformly distributed in the gel medium and antigen is added to a well cut into the gel. As the antigen diffuses from the well, the antigen-antibody combination occurs in changing proportions until the zone of equivalence is reached and a stable lattice network is formed in the gel. The area of the visible ring is compared with standard concentrations of antigens. A variation of this principle is rocket immunoelectrophoresis (Fig. 11-4). The classic Ouchterlony double diffusion technique (Fig. 11-5). performed on a gel medium is used to detect the presence of antibodies and determine their specificity by visualization of lines of identity, or precipitin lines. The reaction of antigen-antibody combination occurs by means of diffusion. The size and position of precipitin bands provide information regarding equivalence or antibody excess. Proteins are differentiated not only by their electrophoretic mobility, but also by their diffusion coefficient and antibody specificity. Although double immunodiffusion produces a separate precipitation band for each antigen-antibody system in a mixture, it is often difficult to determine all the components in a complex mixture. Each line represents one specific protein (Fig. 11-6). Proteins are thus differentiated by their diffusion coefficient and antibody specificity as well as electrophoretic mobility. Antibody diffuses as a uniform band parallel to the antibody trough. If the proteins are homogeneous or of like composition, the antigen diffuses in a circle and the antigen-antibody precipitation line resembles a segment, or arc, of a circle. If the antigen is heterogeneous or not uniform in composition, the antigen-antibody line assumes an elliptical shape. One arc of precipitation forms for each constituent in the antigen mixture. This technique can be used to resolve the protein of normal serum into 25 to 40 distinct precipitation bands. The exact number depends on the strength and specificity of the antiserum used.
Electrophoresis Techniques
Electrophoresis
Upper profile, Distribution characteristic of healthy people. (From Kaplan LA, Pesce AJ: Clinical chemistry: theory, analysis, correlation, ed 5, St Louis, 2010, Mosby.)
Immunoelectrophoresis
Passive Immunodiffusion Procedures
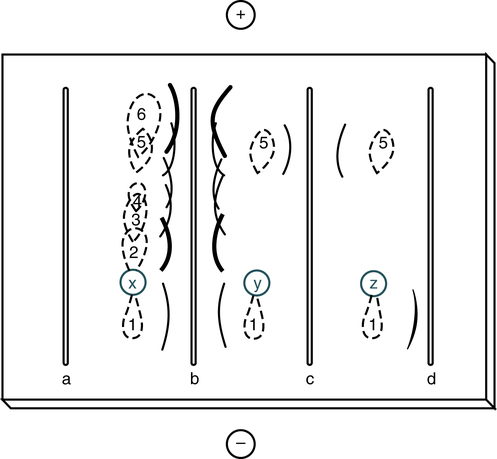
Sample wells are punched in the agar-agarose, sample is applied, and electrophoresis is carried out to separate the proteins in the sample. Antiserum is loaded into the troughs and the gel is incubated in a moist chamber at 4° C (39° F) for 24 to 72 hours. Track x represents the shape of the protein zones after electrophoresis; tracks y and z show the reaction of proteins 5 and 1 with their specific antisera in troughs c and d. Antiserum against proteins 1 through 6 is present in trough b. (From Burtis CA, Ashwood ER, Bruns DB: Tietz fundamentals of clinical chemistry, ed 6, St Louis, 2008, Saunders.)
Principle
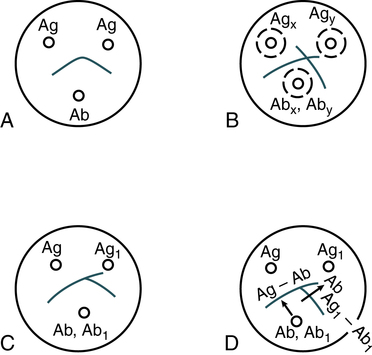
A, Reaction of identity. B, Reaction of nonidentity. C, Reaction of partial identity. D, Scheme for spur formation. Ab, Antibody; Ag, antigen. (From Burtis CA, Ashwood ER, Bruns DB: Tietz fundamentals of clinical chemistry, ed 6, St. Louis, 2008, Saunders.)
Electrophoresis Techniques