First-line series
Second-line series
Unselected patients
INTACT-1, INTACT-2
IDEAL 1 and 2
BR.21
Iressa Survival Evaluation in Lung Cancer
V-15-32
INTEREST
Selection by background
IPASS
Selection by EGFR mutation
NEJ 002
WJTOG 3405
EURTAC-SLCG GECP06/01
OPTIMAL (CTONG 0802)
10.2.1 Unselected Patients
Gefitinib was evaluated in a phase III study (Iressa Survival Evaluation in Advanced Lung Cancer (ISEL)) on NSCLC patients unselected by EGFR status [21]. In total, 1692 patients who were refractory to chemotherapy or intolerant of chemotherapy were randomly allocated to gefitinib treatment (250 mg/day) or placebo plus best supportive care. The primary endpoint, median survival time (MST), was 5.1 months in the placebo group and 5.6 months in the gefitinib group, with no significant differences between the groups (p = 0.087). Therefore, efficacy of gefitinib in unselected NSCLC patients was not indicated. In a preplanned subgroup analysis of the ISEL trial [36], gefitinib was shown to extend survival in Asian patients (MST, 9.5 months vs. 5.5 months; HR = 0.66, p = 0.01). In addition, a covariate analysis of demographic subsets of patients of Asian origin who had been treated with gefitinib showed a survival advantage for never smokers (HR, 0.37; p = 0.0004) and adenocarcinoma patients (HR, 0.54; p = 0.0028).
10.2.2 Selection by Background
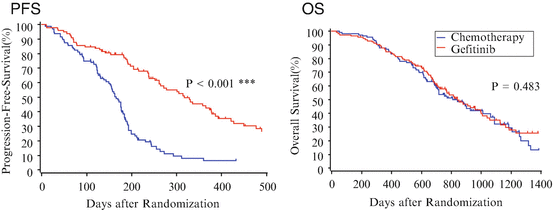
In March 2006, the Iressa Pan-Asia Study (IPASS) was initiated to investigate the effectiveness of gefitinib as a first-line therapy for previously untreated patients in East Asia who had advanced pulmonary adenocarcinoma and who were light- or non-smokers [25]. A total of 1217 NSCLC patients were selected by background and received either gefitinib or carboplatin (CBDCA) plus paclitaxel (PTX). For progression-free survival (PFS), the primary endpoint of this study, an HR of 0.741 was reported (95 % CI, 0.651–0.845) for the gefitinib group. However, since the survival curves for the two groups crossed each other, it was difficult to interpret the HR (Fig. 10.1a). Cox proportional hazards model should be applied where there is a constant relationship between HR and time [37], which is not the case when crossing curves. For example, PFS of gefitinib was variously better, the same, or worse than that of CDBCA + PTX at 12, 6, and 3 months, respectively, indicating changing the HR values in time.
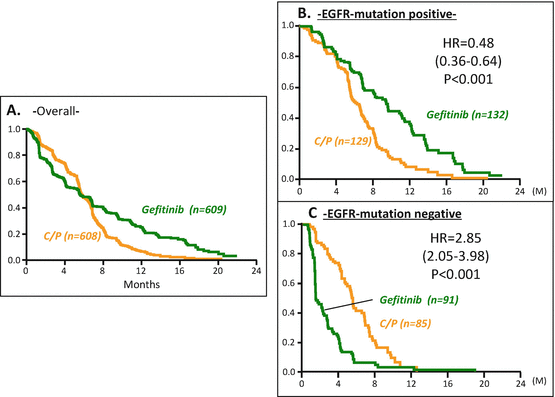

Fig. 10.1
Survival curves in IPASS study. In (a) the survival curves for the two groups crossed each other, indicating no indication for applying Cox model, while in (b, c) the crossing of the survival curves for the two treatment groups disappeared
Although the results regarding the primary endpoint was inconclusive, the importance of the IPASS report is demonstrated in its subset analyses. [25] An EGFR mutation test (amplification mutation refractory system) was performed on tumor samples from 437 of the 1217 patients (36 %). EGFR mutations were identified in 261 patients; PFS in these patients was significantly longer for those who received gefitinib compared to CBDCA+ PTX (HR = 0.48; P < 0.001); by contrast, in the 176 patients who were negative for EGFR mutation, PFS was significantly longer among those who received CBDCA plus PTX (HR = 2.85; P < 0.001). Thus, the subset analyses of IPASS study indicated the possibility of indication for gefitinib treatment in patients who were positive for EGFR mutations.
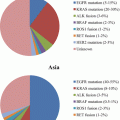
In addition, the biomarkers were compared in the IPASS study for EGFR gene copy number (fluorescent in situ hybridization), EGFR protein expression (immunohistochemistry), and EGFR mutations (direct sequencing) [38]. PFS after gefitinib treatment was significantly longer in patients whose tumors had EGFR mutations (HR, 0.48) and was significantly shorter in patients whose tumors had no EGFR mutations (HR, 3.85). Among the three biomarkers, EGFR mutation was the strongest predictor of PFS and tumor response to gefitinib versus CBDCA plus PTX (Fig. 10.2), while selection of patients who were of Asian origin, had an adenocarcinoma histology, and were light- or non-smokers resulted in producing an EGFR mutation-rich population (60 %, 261 EGFR mutations/437 patients evaluated). If a strategy of selection by background is employed, there is an approximately 40 % risk of including patients for TKI treatment who do not carry EGFR mutations.
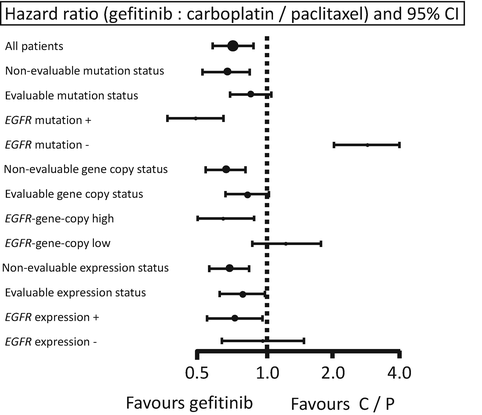

Fig. 10.2
Biomarkers for EGFR-TKI. Forest plot of progression-free survival (PFS) by epidermal growth factor receptor (EGFR) mutation status, gene copy number, and protein expression status. Hazard ratio 1 implies a lower risk of progression or death for patients treated with gefitinib. The size of the point estimate reflects the number of events in the subgroup, with a larger circle indicating more events
10.2.3 Selection by EGFR Mutation
Since the pivotal studies of 2004 reported on the relationship between EGFR mutations and TKI sensitivity, a number of phase II studies in Japan have confirmed the striking response to EGFR-TKIs in patients harboring sensitive EGFR mutations [26–32]. A combined analysis employing these phase II studies, named I-CAMP ( IRESSA Combined Analysis of the Mutation Positives), indicated longer PFS with gefitinib than with standard chemotherapies [39]. In March 2006, at the same time that the IPASS study started, two phase III trials, the North East Japan (NEJ) 002 Study [12] and the West Japan Thoracic Oncology Group (WJTOG) 3405 [33], were initiated to compare gefitinib with standard chemotherapies as a first-line treatment for EGFR-mutated NSCLC (Table 10.1). NEJ 002 confirmed that PFS in the gefitinib group was significantly longer than in the CBDCA plus PTX group (10.8 months versus 5.4 months, HR = 0.30, P < 0.001) (Fig. 10.3). The gefitinib-treated group in WJTOG3405 also had a significantly longer PFS compared to a cisplatin plus docetaxel group, with a median PFS of 9.2 months versus 6.3 months (HR, 0.489, p < 0.0001).
The efficacy of another EGFR-TKI, erlotinib, was investigated in phase III studies of OPTIMAL [34] initiated in August 2008 and EURTAC [35] started in February 2007. OPTIMAL compared the PFS of erlotinib with that by gemcitabine plus carboplatin as the first-line treatment for Chinese patients with advanced EGFR-mutated NSCLC. Median PFS was significantly longer in erlotinib-treated patients than those on chemotherapy (13.1 vs. 4.6 months; HR = 0.16; p < 0.0001). In EURTAC, PFS was also used as the endpoint in a comparison of erlotinib with standard chemotherapy for first-line treatment of European patients with advanced EGFR-mutated NSCLC. The preplanned interim analysis showed that the median PFS was 9.7 months in the erlotinib group, compared with 5.2 months in the standard chemotherapy group (HR = 0·37; p < 0.0001).
A retrospective study by the National Cancer Center Hospital of Japan found that OS became significantly longer among the EGFR-mutant patients treated after gefitinib approval compared to those treated before gefitinib approval (MST, 27.2 vs. 13.6 months, respectively; P < 0.001) [40], indicating the importance of using EGFR-TKIs for EGFR-mutant patients. NEJ 002 [41] and WJTOG 3405 [42] showed identical OS between gefitinib and chemotherapy in first-line treatment of NSCLC patients harboring sensitive EGFR mutations (Fig. 10.3). Almost all patients who were treated with first-line chemotherapy in NEJ 002 and WJTOG 3405 were subsequently given a crossover treatment with gefitinib. Therefore, from the viewpoint of OS, the effect of gefitinib is additive to that of the chemotherapy. With regard to the timing of use of an EGFR-TKI, it is indicated that both first-line and second-line gefitinib are acceptable.
When OS is identical between two arms, then improvement in quality of life (QoL) is the key goal of treatment of NSCLC. NEJ 002 and OPTIMAL presented QoL results [43, 44]. In NEJ 002, QoL of patients was assessed weekly using the Care Notebook [45]; the primary endpoint of the QoL analysis was time to defined deterioration from a baseline on physical, mental, and life well-being scales. Kaplan-Meier probability curves and log-rank tests showed that time to 9.1 % or 27.3 % deterioration in daily functioning significantly favored gefitinib over chemotherapy (HR = 0.43, p < 0.0001, and HR, 0.32, p < 0.0001, respectively) (Fig. 10.4). It was indicated that QoL was maintained much longer in patients treated with gefitinib than in those treated with standard chemotherapy. In OPTIMAL, the Functional Assessment of Cancer Therapy (FACT) measuring system showed that, in comparison to the gemcitabine plus carboplatin group, the erlotinib group had a clinically relevant improvement in QoL, as assessed by scores on the FACT-L (73 % vs. 29.6 %; odds ratio (OR) = 6.9; p < 0.0001), the LCSS (75.7 % vs. 31.5 %; OR = 6.77; p < 0.0001), and the TOI (71.6 % vs. 24.1 %; OR = 7.79; p < 0.0001). These QoL results conclusively indicate that EGFR-TKIs should be considered as the standard first-line therapy for advanced EGFR-mutated NSCLC despite the lack of any survival advantage.
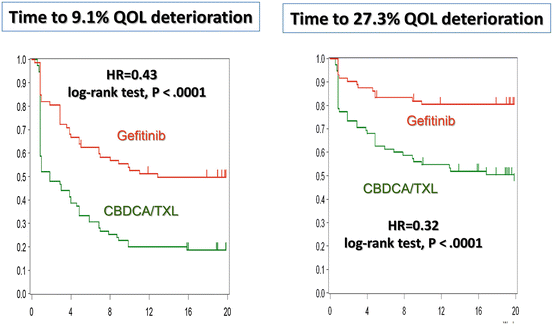

Fig. 10.4
Daily functioning in NEJ 002. Time to 9.1 and 27.3 % QoL deterioration for daily functioning are shown (Oizumi et al. [43])
10.2.4 EGFR-Mutated Patients with Poor Performance Status and Advanced Age
The multicenter phase II NEJ001 study was undertaken to investigate the efficacy and feasibility of gefitinib treatment for advanced NSCLC patients harboring EGFR mutations, but who were ineligible for chemotherapy due to poor performance status (PS) [11]. The overall response rate in this patient group was 66 %, and median PFS and MST were 6.5 months and 17.8 months, respectively. PS improved by 79 % (p < 0.00005); in particular, 14 (68 %) of 22 patients improved from PS ≥ 3 at baseline to PS 0 or 1 (Fig. 10.5 ). Thus, the “Lazarus Response” (in which Jesus restored Lazarus to life 4 days after his death) was observed in treatment-naïve, poor PS patients with NSCLC and EGFR mutations [46]. In patients with sensitive EGFR mutations but with extremely poor PS (suspected MST less than 4 months with best supportive care (BSC)), the difference in benefit with or without gefitinib treatment was so marked that a randomized phase III study to compare gefitinib to BSC alone may not be justified. This was the first occasion on which changes in treatment guidelines were provoked by a phase II study of NSCLC. Since previously there has been no standard treatment for these patients with short life expectancy other than BSC, examination of EGFR mutations as a biomarker is also strongly recommended in this patient population.
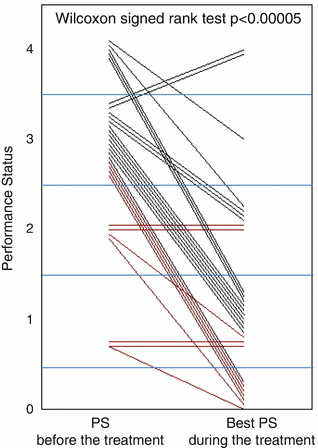

Fig. 10.5
Change of performance status of each patient during gefitinib treatment in NEJ 001. Each line shows the change of performance status (PS) of a patient from baseline to best status during the treatment. A clinically valuable improvement in 68 % of patients was observed, i.e., they improved from PS 3–4 at baseline to PS 0–1
With regard to so-called “fit” elderly patients harboring EGFR mutations, the NEJ003 phase II study [13] investigated patients with a chemotherapy-naïve history, a median age of 80 years (range, 75–87 years), and a PS 0–1, who were treated with gefitinib as a first-line treatment. The response rate was 74 %, and the median PFS and OS were 12.3 months and 33.8 months, respectively. Considering its strong antitumor activity and mild toxicity, first-line gefitinib may be preferable to standard chemotherapy in this population.
10.2.5 Interstitial Lung Disease (ILD) After Gefitinib and Erlotinib Treatment
Common adverse events associated with EGFR-TKI treatments are diarrhea, skin rashes, and nausea; these are mild in severity and manageable. However, EGFR-TKI agents can induce ILD, which has the potential to be fatal. The incidence of ILD during EGFR-TKI treatments was reported to range from 1 to 5.4 %. The US Food and Drug Administration (FDA) reported 1 % worldwide incidence of ILD in 50,000 patients who received gefitinib in 2003 [47]. The incidence of ILD in Japanese populations was reported to be 4.0 % (95 % confidence interval, 3.0–5.1 %) [48]. These suggest that the incidence of ILD during EGFR-TKI treatments varies among Japanese and non-Japanese populations.
Risk factors for ILD have been identified in several Japanese studies: preexisting pulmonary fibrosis, poor PS, prior thoracic irradiation, male, smoking, older age (>55 years), recent NSCLC diagnosis, reduced normal lung on computed tomography scan, and concurrent cardiac disease [48]. Furthermore, a Korean study identified lower albumin levels, which might be related to poor PS, as a risk factor [49]. However, the mechanism by which EGFR-TKI may cause ILD is still unclear. Hagiwara K et al. focused on MUC4, a mucin protein encoded by the MUC4 gene, and reported that specific polymorphisms might be associated with the risk of EGFR-TK-induced ILD (patented).
EGFR-TKI-induced ILD is often life-threatening. The main treatments for EGFR-TKI-induced ILD are immediate discontinuation of the EGFR-TKI and systemic corticosteroids; however, there have been no controlled trials to evaluate the efficacy of these strategies. Other supportive treatments include supplemental oxygen, empirical antibiotics, and mechanical ventilation. However, the mortality for gefitinib-induced ILD is approximately 30–40 % [48].
10.3 Second- and Third-Generation EGFR-TKI
10.3.1 Second-Generation EGFR-TKI: Afatinib
Second-Generation EGFR-TKIs
First-generation EGFR-TKIs were designed to inhibit ATP binding to wild-type EGFR tyrosine kinase via reversible competitive binding. By contrast, second-generation EGFR-TKIs, such as afatinib, neratinib, and dacomitinib, were designed to covalently bind to ERBB receptor family members and irreversibly block their enzymatic activity. Second-generation EGFR-TKIs inhibit tyrosine kinase activity in EGFR/ERBB1 and also in HER2/ERBB2 and HER4/ERBB4, which contain an electrophilic group capable of a Michael addition to conserved cysteine residues within the catalytic domains of EGFR (Cys797), HER2 (Cys805), and HER4 (Cys803). The precise role of HER2 and HER4 in lung cancer remains unclear; the conformation of HER2 resembles a ligand-activated state and may favor pathogenic EGFR signaling after formation of a heterodimer with EGFR [50]. The Kd of afatinib for wild-type EGFR, exon19 EGFR, and L858R EGFR is 0.25 nM, 0.11 nM, and 0.2 nM, respectively (Table 10.2) [51]. Afatinib shows comparable ability to inhibit EGFR tyrosine kinases with uncommon mutations, such as G719X or L861Q. Notably, the Kd of afatinib for EGFR with both L858R and T790M is 1.1 nM, and antitumor activity was estimated for EGFR acquire T790M by these enzymatic analyses.
Table 10.2
A quantitative dissociation constant (Kd (μM)) of afatinib, erlotinib, and gefitinib for ERBBB family kinases
Kinases | Afatinib | Erlotinib | Gefitiib |
|---|---|---|---|
EGFR | 0.25 | 0.67 | 1 |
EGFR(E746 = A750del) | 0.11 | 0.48 | 0.54 |
EGFR(G719C) | 0.1 | 0.85 | 2 |
EGFR(G719S) | 0.19 | 0.52 | 1.1 |
EGFR(L747 = E749del,A750P) | 0.14 | 0.52 | 0.57 |
EGFR(L747 = S752del,P753S) | 0.12 | 0.47 | 0.57 |
EGFR(L747 = T751del,Sins) | 0.12 | 0.35 | 0.52 |
EGFR(L858R) | 0.2 | 0.97 | 0.94 |
EGFR(L858R,T790M) | 1.1 | 190 | 140 |
EGFR(L861Q) | 0.23 | 1.2 | 1.4 |
EGFR(S752 = I759del) | 0.14 | 1.6 | 0.98 |
EGFR(T790M) | 0.61 | 140 | 40 |
ERBB2 | 5 | 2900 | 3500 |
ERBB3 | 4500 | 1100 | 790 |
ERBB4 | 6.3 | 230 | 410 |
Antitumor Activity of Afatinib in Preclinical Analyses
Cell-based assays showed that afatinib suppresses EGFR tyrosine kinase activity for a longer time than the reversible first-generation EGFR-TKIs; this effect is due to the irreversible suppression of kinase activity that continues until the cancer cells synthesize new EGFR [52]. The effective concentration of afatinib is one to two orders of magnitude below those needed for inhibition of colony formation by erlotinib in soft agar assays of tumor cells harboring EGFR mutations [53]. Although in vitro assays have shown the potency of afatinib (IC50 of 9–10 nM) against cancer cells carrying the EGFR L858R/T790M double mutation [54], a xenograft model showed that afatinib alone did not exhibit enough therapeutic efficacy against tumor cells harboring the exon20 T790M mutation. An additional anti-EGFR mAb, cetuximab, was required to overcome EGFR-TKI resistance in cells with T790M [55].
Clinical Benefits of Afatinib for Common EGFR Mutations
Two phase III trials, LUX-Lung 3 and LUX-Lung 6, have been conducted to evaluate the efficacy of first-line afatinib on overall survival of patients who have advanced lung adenocarcinoma harboring EGFR-activating mutations [56, 57, 58]. It is noteworthy that overall survival was significantly longer for patients with EGFR exon19 del in the afatinib group than in the chemotherapy group in both trials (Fig. 10.6). By contrast, there were no significant differences between treatments for patients with EGFR L858R tumors. Four randomized phase III studies conducted to evaluate first-generation EGFR-TKIs, such as gefitinib and erlotinib, for advanced lung cancer patients harboring EGFR-activating mutations could not demonstrate superiority of EGFR-TKI to platinum doublet chemotherapy in overall survival of patients with either exon19 del or L858R; in these studies, most patients who were assigned to the chemotherapy group received first-generation EGFR-TKIs as a second-line treatment. It is likely that EGFR-TKI and platinum doublet chemotherapy are mutually non-cross resistant and that the sequence of the two treatments makes no differences to antitumor efficacy. In contrast, in LUX-Lung 3 and LUX-Lung 6, most patients who were assigned to chemotherapy received first-generation EGFR-TKIs but not second-generation EGFR-TKIs as a second-line treatment. Kato et al. analyzed the Japanese subgroup in LUX-Lung 3 and demonstrated that first-line afatinib produced a consistent and significant improvement in overall survival results in patients with exon19 del but not in patients with L858R, even though most patients (93.5 %) received subsequent first-generation EGFR-TKI therapy [59]. Thus, LUX-Lung 3 and LUX-Lung 6 indicated a possible superiority of afatinib to first-generation EGFR-TKIs in EGFR exon19 del patients and showed for the first time biologically significant differences between two common activating EGFR mutations, exon19 del and L858R.
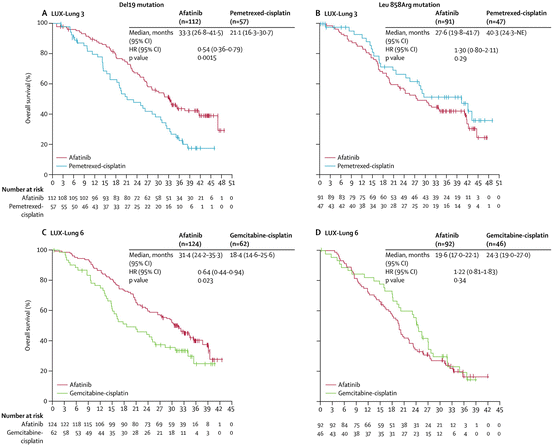

Fig. 10.6
Overall survival in lung cancer patients harboring EGFR exon19 del or L858R who were treated in LUX-Lung 3 and LUX-Lung 6. Overall survival of EGFR exon19 del-positive disease patients in (a) LUX-Lung 3 and (c) LUX-Lung 6. L858R-positive disease patients in (b) in LUX-Lung 3 and (d) LUX-Lung 6. HR, hazard ratio. NE not estimable
Differences Between Exon19 Del and L858R
It has been established that wild-type EGFR is primarily driven by ligand-binding-induced extracellular domain dimerization. After extracellular dimerization, a critical step in EGFR activation is the formation of an asymmetric dimer of kinase domains, in which the C-terminal lobe of one kinase domain (donor) and the N-terminal lobe of another kinase domain (acceptor) associate (Fig. 10.7a). EGFR with L858R preferentially assumes the acceptor role and requires a wild-type EGFR donor for superacceptor activity (Fig. 10.7b) [60, 61]. By contrast, EGFR with an exon19 del is active as either acceptor or donor and is oncogenic even in the absence of dimerization (Fig. 10.7c) [62]. Overall, the oncogenic signaling of exon19 del is dimerization independent and that of L858R is dependent on heterodimer formation with wild-type EGFR. It is still uncertain why afatinib and first-generation EGFR-TKI show different antitumor activities for lung cancer patients harboring exon19 del EGFR. It is likely that EGFR with exon19 del has a higher oncogenic potential and requires stronger inhibition by EGFR-TKI for optimal antitumor efficacy.
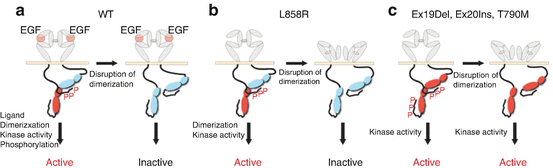

Fig. 10.7
Proposed model of cellular transformation by wild-type (a) EGFR and oncogenic mutations (b, c)
Clinical Benefits of Afatinib for Uncommon Mutations
Approximately 10 % of lung cancer patients with mutated EGFR harbor uncommon mutations, such as G719X, L861Q, or rare mutations. However, there is a paucity of data regarding the sensitivity of these tumors to EGFR-TKI. Yang et al. [[63] ]collected data from LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6 and conducted a post hoc analysis to assess antitumor efficacy of afatinib for uncommon mutations. As estimated from the affinity data for the inhibition of enzymatic activity of mutated EGFR, afatinib demonstrated significant antitumor activity against lung cancer with uncommon EGFR mutations, especially G719X, L861Q, and S768I.
Antitumor Effect of Afatinib for EGFR T790M in Clinical Trials
Although afatinib showed antitumor activity in cell-based assays, it had to be combined in vivo with anti-EGFR mAb to exhibit antitumor efficacy against cancer harboring EGFR T790M [64, 65]. One of the reasons why the in vitro antitumor activity could not be translated into in vivo antitumor efficacy is that a plasma concentration sufficient to inhibit EGFR T790M could not be obtained because nonselective inhibition of wild-type EGFR by afatinib resulted in intolerable adverse events.
Adverse Events with Afatinib
The most common grade 3 or 4 adverse events related to afatinib treatment were rash or acne, diarrhea, paronychia, and stomatitis or mucositis. Compared to first-generation EGFR-TKIs, afatinib induced grade 3 or 4 skin rashes, acne, stomatitis, and diarrhea more frequently because of its higher affinity to wild-type EGFR. The severity of these common adverse events depends on afatinib plasma concentration. By contrast, the frequency of interstitial lung disease in patients treated with afatinib is similar to that in patients treated with first-generation EGFR-TKIs [56, 57]. Grade 3 or 4 transaminase elevation was less frequent in patients treated with afatinib than in patients treated with gefitinib, because afatinib is not metabolized by cytochrome enzymes in the liver.
10.3.2 Third-Generation EGFR-TKI
Third-Generation EGFR-TKI
Although second-generation EGFR-TKIs show stronger activity for inhibiting mutated EGFR tyrosine kinases by forming irreversible covalent bonds, the nonselective inhibition of wild-type EGFR tyrosine kinase results in adverse events such as skin rashes, acne, stomatitis, and diarrhea. Furthermore, the acquisition of a T790M mutation lowers the affinity of EGFR-TKIs for mutant EGFR, and the EGFR-TKI concentrations needed to inhibit tyrosine kinase activity are not achievable due to toxicity related to nonselective inhibition of wild-type EGFR. Third-generation EGFR-TKIs are designed to inhibit EGFR tyrosine kinase harboring activating mutations and T790M through the formation of irreversible bonds while sparing the activity of wild-type EGFR tyrosine kinase. WZ4002 was the first agent to be published. Rociletinib (CO1686), which is closely related to WZ4002, is in clinical trials. Osimertinib (AZD9291) and HM61713 are other third-generation EGFR-TKIs that have progressed to clinical trials [66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree