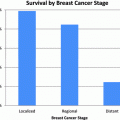
Fig. 1
Comparative effectiveness topics
1.1 Prostate Cancer Screening
Prostate cancer screening is highly controversial. The United States Preventative Services task force gave prostate cancer screening with PSA a D rating [1]. Conversely, the American Urological Society continues to endorse PSA based screening after informed decision making with patients [2]. Here, comparative effectiveness research has been performed to help inform the debate. Two large randomized studies, the Prostate Lung Colorectal and Ovarian (PLCO) trial and European Randomized Study of Screening for Prostate Cancer (ERSPC), came to conflicting conclusions about the efficacy of PSA based screening for preventing death from prostate cancer. The PLCO study showed no benefit to men in the screening arm versus the non-screening arm of the study at 7–10 years of follow up [3]. ERSPC showed that men in the PSA screening group had a 20 % reduction in prostate cancer mortality at a median of 9 years of follow up [4]. Controversy about PSA based screening continues due to the large number of men diagnosed with potentially non-lethal prostate cancer. Since most men diagnosed with prostate cancer in the United States receive treatment [5], a real risk of overtreatment exists. The risks of overdiagnosis and overtreatment are what led to the D rating from the USPSTF [1].
Screening could be improved through development of new tests to improve upon or replace PSA. New approaches, including PCA-3 [6], TMPRESS-erg fusion [7], and other genetic variations [8] have been explored as diagnostic tests for prostate cancer. The Early Disease Research Network is involved in efforts to improve these alternative tests and bring them to the point of clinical use. Should the results be promising for one or more of the markers, they will need robust CER efforts to determine which marker should be used in what screening situation. For instance, PCA-3 has FDA approval for use in the setting on an elevated PSA with a prior negative prostate biopsy to evaluate the need for a subsequent biopsy. As other markers become available, they could be directly tested against the existing approved marker in this setting. Additionally, studies are needed comparing new markers with PSA in unscreened populations. Currently, most new markers are tested as adjunct to PSA based screening. A challenge will be finding unscreened populations in which to perform this testing.
2 Treatment of Localized Disease
2.1 Observation, Active Surveillance, and Active Treatment of Prostate Cancer
With the high risk of over diagnosis of prostate cancer with SPA based screening, efforts have moved to changing the rates of overtreatment of disease that might not actively harm patients. Decreasing overtreatment requires comparative effectiveness research showing non-treatment of prostate cancer is as effective as active intervention. However, two studies of watchful waiting versus prostatectomy show some advantage to prostatectomy in higher risk men. The Scandinavian Prostate Cancer Study Group (SPCG-4) showed a mortality advantage to prostatectomy [9]. These men were typically diagnosed prior to the PSA era, and had more extensive disease than currently seen in practice. The Prostate Intervention Versus Observation Trial (PIVOT) study compared active intervention versus observation in patients treated in the Veterans Administration hospital system. Patients with low risk prostate cancer received no benefit from intervention, whereas men with intermediate or high risk disease appeared to receive some benefit [10]. Important criticisms against this study were the high background mortality rate in the study. Despite attempting to screen for healthy patients, the median survival for patients in the observation and intervention groups was only 10 years. The applicability of these results to other setting remains controversial.
Both SPCG-4 and PIVOT compared observation, not active surveillance, with treatment. Active surveillance differs from observation because men are followed regularly with PSA and biopsy and intervention instituted if higher risk disease develops during observation. No studies are available comparing active surveillance to intervention in a robust manner. Two trials, START and SPIRIT, were stopped due to poor enrollment. Only one in six patients consented for enrollment at a major center despite extensive patient education efforts [11]. An ongoing trial in the United Kingdom, ProtecT, will attempt to answer the role of active surveillance versus active treatment. This study enrolled over 3,000 participants and randomized them to prostatectomy, radiation therapy, or active surveillance [12]. Quality of life and survival results are expected in 2015, and may help guide decision making for men with prostate cancer.
2.2 Comparative Effectiveness of Prostate Cancer Therapies
Once men have chosen active intervention for prostate cancer, little comparative effectiveness evidence exists to guide them in selection of treatments. No trial has been published randomizing men to radiation or surgery, a deficit the ProtecT trial will correct. As such, all comparisons between the modalities have been observational studies. The PCOS trial provided early information about the side effects and quality of life after treatment by the different modalities, and now has shown a survival advantage for men treated with radical prostatectomy over those treated with radiation therapy [13]. Additional quality of life data has come from the PROSQA study showing distinct patterns to urinary and sexual function among men treated with prostatectomy, external-beam radiation therapy, and brachytherapy [14].
Despite these past efforts in prospective patient reported outcomes, major gaps remains in the understanding of efficacy of surgery versus radiation. Since the enrollment of men in the PCOS study in the 1990s, substantial changes have occurred in radiation therapy administration, including better image guidance, higher doses of radiation provided, and use of proton beam therapy. A new observational study, CEASAR attempts to rectify this knowledge gap [15]. The study enrolled over 3,000 men in a prospective cohort. Detailed information on demographics, treatment preferences, treatments received, and functional outcomes (urinary, sexual, and bladder) are being assessed. The study will provide updated information on the comparative effectiveness of the many different treatments for localized prostate cancer. Initially, these results will focus on differences in symptoms after treatment and the long term resolution or progression of these symptoms. Future plans include longer follow up to provide information on survival differences between different treatment modalities.
ProtecT and CEASAR will provide excellent data to help resolve controversies about active surveillance versus active treatment. However, even these well designed studies cannot answer all of the relevant questions regarding treatment versus active surveillance and surgery versus radiation. Further investigations based on the new findings from these studies will be needed. A concern will also be the applicability of the results of the ProtecT study to African–Americans. CEASAR has a broader enrollment, but the long term follow up for efficacy is planned, but not funded per their recent publication [15].
2.3 Comparative Effectiveness of Surgical Approaches for Prostate Cancer
Once patients make their decision regarding treatment modality, a new set of clinical questions emerges. Observational studies have addressed minimally invasive versus open approaches to radical prostatectomy. Early studies showed improved length of stay with minimally invasive approaches and lower use of blood transfusions. These improvements were balanced by higher rates of incontinence and erectile dysfunction [16]. Further investigations have failed to show significant differences in laparoscopic radical prostatectomy versus the use of robotic assistance [17]. Despite the paucity of data, most prostatectomies performed in the United States are done using a robotic assisted technique.
The spread of robotic prostatectomy reflects a common problem in surgical innovation. New technology disseminates before any comparative effectiveness studies show a benefit to the new technology. Once the technology has saturated the market, designing high quality comparative effectiveness studies becomes difficult. Here, the possibility of randomized controlled trials is unlikely due to patient preference and direct to consumer marketing. Unique prospective cohorts, with well defined data collection and study parameters, are needed to answer important comparative effectiveness questions.
2.4 Comparative Effectiveness of Radiation Therapy Modalities
Radiation therapy has an additional set of treatment choices, where the options lack solid comparative effectiveness data (Table 1). Current guidelines recommend 3-D conformal or intensity modulated radiation therapy with image guidance for external beam radiation therapy with primary brachytherapy an alternative for patients with low risk cancers [18]. Aside from the previously mentioned studies comparing functional outcomes between brachytherapy and external beam radiation therapy, good studies exploring cancer control with the multiple different radiation modalities are lacking. For instance, no comparative effectiveness studies were done comparing conformal external beam radiation with IMRT [19], yet IMRT is the dominant form of external beam therapy used in the United States [20]. In a population based study using SEER-Medicare data, IMRT showed less gastrointestinal morbidity and fewer hip fractures than conformal radiation [21]. However, IMRT was associated with increased risk of erectile dysfunction. This study also compared IMRT to proton therapy and found less gastrointestinal complications with IMRT. The lack of data comparing different modalities is one place the CEASAR study may provide additional information [15]. However, one study alone cannot address all of the controversies in radiation options, and other well designed prospective or randomized studies will be needed.
Table 1
Treatments and comparisons needed in radiation therapy for prostate cancer
Option | Comparison needed |
|---|---|
Intensity modulated radiation therapy | Conformal beam radiation therapy |
Brachytherapy | IMRT or CBT |
Proton beam therapy | IMRT, CBT, or Brachytherapy |
2.5 Recurrent and Metastatic Prostate Cancer
For years the treatment of metastatic prostate cancer was simple and limited by available medications. Men with metastatic prostate cancer were treated by androgen deprivation, initially through orchiectomy and later through luteinizing hormone receptor agonists. After failure of these methods, men received best supportive care with no evidence that further androgen manipulation or chemotherapy helped improve outcomes. In the past ten years, treatment options for metastatic prostate cancer have grown enormously. The proper sequencing and use of these medications remains an area of active research. In this section, we discuss three important topics for comparative effectiveness research: how should men with a rising PSA after definitive therapy be treated, what medication should men with asymptomatic or mildly symptomatic metastatic disease be treated with, and what treatment should be provided to men with symptomatic metastatic disease.
A central unresolved question in prostate cancer therapy is when to initiate androgen deprivation therapy. This question exists both in patients who have PSA recurrence after prior radiation or surgical therapy, and in patients who are not candidates for definitive therapy, but have a rising PSA without evidence of metastatic disease. No studies currently address these issues. From prior case series, the time period from PSA detection to development of metastatic disease is estimated to be 5 years [22], and the PSA velocity during recurrence is an important predictor of metastatic disease and death [23]. NCCN guidelines acknowledge the lack of evidence about when to initiate ADT. Resolution of this issue will require unique data sets and examination, and are unlikely to be resolved with randomized trials.
Documentation of metastatic disease is another area of controversy. Since prostate cancer primarily recurs in bone, assessment for metastatic disease with 99mTc-medronate bone scans has been central for diagnosis. A newer modality, 18F-NaF PET has been developed. This technology has increased sensitivity for detection of bone metastases [24]. However, how this increased sensitivity impacts management of men with metastatic prostate cancer remains unresolved.
Once metastatic disease is documented, men with prostate cancer have multiple options for management. A continuing controversy involves the use of combined androgen blockage with a luteinizing hormone releasing hormone agonist combined with an anti-androgen versus LHRH agonist monotherapy. Furthermore, the role of LHRH antagonists versus agonists remains to be identified.
When the PSA rises during ADT therapy, patients are considered to have castration-recurrent prostate cancer. Treatment options vary based on the severity of patient symptoms. For patients with asymptomatic CRPC, level 1 evidence supports the use of Sipilucel-T and Abiraterone [25, 26]. A randomized trial of enzalutamide in this setting has also been completed and the results have been presented but publication of the results is still pending. For patients who are symptomatic, docetaxel and radium-223 have level one evidence supporting their use [27–29]. In addition, the trials with abiraterone and enzalutamide included men who were mildly symptomatic, while the trials with docetaxel included some asymptomatic men. With the substantial overlap between agents and the studies supporting the agents, no good evidence exists to support the sequencing of agents. Trials comparing the agents and alternative sequencing of agents are needed.
3 Bladder Cancer
For most patients with low grade and non-muscle invasive bladder cancer, treatments are routine and control the cancer with little risk of progression to metastatic disease and death. Most controversy for these patients exists in the proper sequence and amount of surveillance care patients receive. Among higher risk patients with high grade or muscle invasive disease, risk or recurrence and death is high. It is in these patients that current treatment options are limited and extensive controversies exist.
3.1 Non-muscle Invasive Bladder Cancer
Low grade, non-muscle invasive, bladder cancer is characterized by a high recurrence rate with a low rate of progression to muscle invasive disease or development of metastatic disease. These patients are managed by resection of the tumor through a transurethral approach followed by surveillance regimens. These surveillance regimens have proven controversial, with few patients receiving recommended care [32], and adherence with recommended care showing no benefits with overall or cancer specific survival [33]. Despite these controversies, no randomized trials exist to guide care. Practice continues based on best practices, and no trials are currently under way to address the issue.
One issue that has been well investigated in the non-muscle invasive bladder cancer population is the use of intravesical chemotherapy after resection. A single dose of intravesical chemotherapy increased recurrence free survival by 38 % in a recent meta analysis [34]. Medications used included mitomycin-c, epirubicin, peplomycin, THP-doxorubicin, and gemcitabine, with mitomycin-c and epirubicine showing the greatest efficacy compared to placebo or not active intervention. However, no CER study has been performed comparing one agent to another, making this an area where CER research could be employed.
3.2 Follow-up Care After Definitive Bladder Cancer Therapy
Bladder cancer patients who receive a radical cystectomy remain at high risk for recurrence and death from their disease. For these patients, the ideal follow up regimen is now known. Recommended follow up tests include CT or MRI scans [35], trans-rectal ultrasound [36], no imaging [37], voided cytology [35, 38] and urethral wash cytology [35, 38–40]. The performance of these follow up studies in patients treated with neoadjuvant or adjuvant chemotherapy is unknown [41, 42]. A recent analysis found urine testing after cystectomy and doctor visits positively impacted patient survival, but imaging tests had no impact [43]. While the observational data helps point towards beneficial modalities of therapy, further research comparing the effectiveness of different follow up patterns is needed to positively impact the outcomes for patients.
3.3 Comparative Effectiveness of Radiation and Surgery for Muscle Invasive Bladder Cancer
A final area of controversy is in bladder sparing protocols for muscle invasive bladder cancer versus radical cystectomy. Overall, curative therapy is used by only a fraction of patients with muscle invasive bladder cancer [44]. Trimodality therapy, combining transurethral resection of the tumor, radiation therapy and radio sensitizing chemotherapy provides an additional option to patients for cure of muscle invasive bladder cancer. Results of this therapy are comparable to those of radical cystectomy with five year survival rates of 48–65 % and bladder preservation rates of 70 % [45]. However, in the absence of comparative studies, the true effectiveness comparing trimodality therapy to radical cystectomy remains unknown. Issues of patient selection for each therapy and standardization of both surgical and radiation management need to be explored.
The comparative effectiveness of surgical versus radiation therapy for muscle invasive bladder cancer fits into a framework of decision making for patients with muscle invasive bladder cancer (Fig. 2). The initial treatment decision for surgery versus radiation or observation is followed by a decision regarding the use of neoadjuvant chemotherapy, and finally a set of variables related to the quality of the surgery. Each of these points requires robust research to answer questions about the effectiveness of the available interventions. For example, groups are trying to refine patient selection for neoadjuvant chemotherapy referral [46]. Other efforts are underway comparing the quality and effectiveness of robotic and open approaches to surgery. Finally a randomized clinical trial (S1011) assessing the extent of lymph node dissection is accruing patients in a cooperative group trial. Such efforts will help answer critical questions about the appropriateness of different surgical techniques and are needed for multiple aspects of the surgical management of bladder cancer.
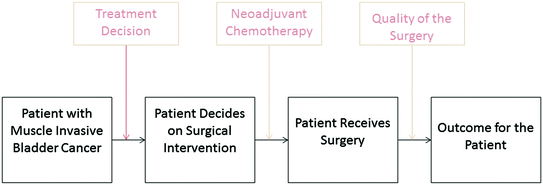

Fig. 2
Treatment decisions in muscle invasive bladder cancer
4 Kidney Cancer
Recent expansions in the therapeutic options for patients with kidney cancer have raised important questions related to clinical effectiveness. Management of localized disease, metastatic disease with the primary kidney tumor in place, and metastatic disease that developed after prior nephrectomy are disease states where comparative effectiveness research is needed. In this next section, we discuss these controversies and the limitations in current understanding.
4.1 Comparative Effectiveness of Surgical Methods for Kidney Cancer
Localized kidney tumors are typically managed by surgical resection. Multiple alternatives, ranging from laparoscopic, open, and robotic approaches to surgery, exist. Additionally, the entire kidney can be removed versus just the portion containing the cancer. Further options for local control involve percutaneous or laparoscopic cryoablation of the tumor or radiofrequency ablation of the tumor. In addition to local control, many centers advocate observation of small renal masses, especially in the elderly or patients with extensive comorbidity. Some CER has been performed to inform patients and surgeons on the relative risks and benefits of each approach to management of localized kidney cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree