Author, year
Complications due to primary (%)
Benoist, 2005
15
Cellini, 2010
67
Galizia, 2008
30
Karoui, 2011
19
McCahill, 2012
14
Muratore, 2007
8.6
Ruo, 2003
29
Scoggins, 1999
8.7
Seo, 2010
19.9
Tebutt, 2003
9.8
Resection of the primary has its own risks. The risk of mortality with resection of the primary has been found to be between 1.6 and 4.6 % [19, 20]. Resection of the primary tumor delays initiation of systemic therapy, particularly if postoperative complications occur, potentially allowing progression of metastases. Rarely, patients may still be at risk for complications from the primary tumor, such as obstruction or perforation, if there is recurrence of the tumor either at an anastomosis or at another site in the bowel. In fact, a recent Cochrane review concluded there was no significant reduction in the risk of complications when surgery as the initial therapy was compared to chemotherapy and/or radiation as the initial therapy [21]. These findings have lead several authors to recommend avoidance of resection for asymptomatic primary tumors, [18, 20, 22, 23] while others recommend resection, particularly for patients who are low-risk for surgery from a medical standpoint [24, 25].
Resection of the primary could also theoretically slow the progression of the disease by debulking the tumor, allowing chemotherapy to work more effectively on the remaining disease. Many studies have examined the possibility of a survival benefit with resection of the primary tumor, but these have been hampered by the strong influence of patient selection since these were retrospective studies. Often patients who were in better medical condition or those who were thought to have a possibility of cure with from a combination of resection and chemotherapy were chosen for resection, leading to a bias toward much more advanced disease in the nonresected subjects. Results of such studies have varied widely. For studies limited to patients with asymptomatic primary tumors and unresectable metastatic disease, some studies have shown a survival benefit while the majority have not (Table 2). The authors of these studies have been split on whether resection of the primary is beneficial. Some have advocated for this approach [26] whereas others have not [20, 27–29]. If the criteria are expanded so that studies that include symptomatic primaries or resectable metastatic disease are included (Table 3), the picture becomes even more clouded. In these studies, it is difficult to determine how much of the improvement in survival, if demonstrated, could be due to patients who are curable. Some multivariate analyses have found tumor resection to be an independent predictor of survival [30–32] while others have not [33]. A Cochrane review on the subject concluded there is no consistent improvement in overall survival with resection of the primary tumor [21].
Table 2
Case series evaluating resection of primary in unresectable metastatic colon and rectal cancer with asymptomatic primary tumors
Author, year | Number of patients | Follow up, months | Survival, months | Comments |
|---|---|---|---|---|
Benoist, 2005 | Difference in survival not significant | |||
Resection of primary | 32 | Not stated | 2-year OS: 44 % | |
No resection | 27 | 2-year OS: 41 % | ||
Galizia, 2008 | p = 0.03 for difference in survival | |||
Resection of primary | 42 | 16 | 15 | |
No resection | 23 | 12 | 12 | |
Ruo, 2003 | p < 0.001 for difference in survival | |||
Resection of primary | 127 | >80 % followed until death | 16 | |
No resection | 103 | 9 | ||
Scoggins, 1999 | Difference in survival not significant | |||
Resection of primary | 66 | Not stated | 14.5 | |
No resection | 23 | 16.6 | ||
Seo, 2010 | Survival different in univariate but not multivariate analysis | |||
Resection of primary | 144 | 49 | 22.0 | |
No resection | 83 | 14.0 | ||
Tebutt, 2003 | Difference in survival not significant in multivariate analysis | |||
Resection of primary | 280 | 30 | 14.0 | |
No resection | 82 | 19 | 8.2 |
Table 3
Studies evaluating resection of primary in metastatic colon and rectal cancer, resectable metastatic disease and symptomatic primaries included
Author, year | Number of patients | Follow up, months | Survival, months | Comments |
|---|---|---|---|---|
Cellini, 2010 | Difference in survival not significant | |||
Staged primary and liver | 13 | 23 | 50 | |
Synchronous primary and liver | 30 | 54 | ||
Primary only | 22 | 32 | ||
No resection | 9 | 37 | ||
Chan, 2010 | ||||
Resection of primary | 286 | Variable | 14 | |
No resection | 125 | 6 | ||
Chew, 2012 | Patients routinely received primary resection unless contraindication | |||
Resection of primary | 696 | 9 | 1-year cancer-spec.: 48.7 % | |
No resection | 22 | 1-year cancer-spec.: 9 % | ||
Cook, 2005 | Analysis of the SEER database | |||
Resection of primary | 17,658 | Not stated | 11 colon, 16 rectal | |
No resection | 9,097 | 2 colon, 6 rectal | ||
Karoui, 2011 | Resection independent predictor of survival in multivariate analysis | |||
Resection of primary | 85 | 19.7 | 30.7 | |
No resection | 123 | 21.9 | ||
Konyalian, 2007 | Resection independent predictor of survival in multivariate analysis | |||
Resection of primary | 62 | Not stated | 12.3 | |
No resection | 47 | 4.5 |
Thus, retrospective data have been very limited in their ability to allow reliable conclusions about the role of resection of the primary due to issues with patient selection. Ideally, a randomized controlled trial could be done among patients with asymptomatic primaries and unresectable metastatic disease who are medically fit to undergo resection, thus eliminating these patient selection factors that have plagued retrospective studies. However, recruitment for such studies has been difficult, with at least two studies being closed due to lack of recruitment [34, 35]. This difficulty with recruitment likely relates to patients being unwilling to undergo surgery for unclear benefit, or care providers encouraging them to have resection due to the perceived risk of complications from the primary. There is currently another randomized controlled trial on this subject underway, [36] which one hopes will provide definitive data as to whether primary tumor resection is associated with decreased complications or lengthened survival.
Other important issues in these cases are quality of life and patient preferences. Since surgery can be associated with short-term decreases in quality of life, this could be a major consideration in patients who likely have a limited life expectancy. Patient preferences in this area could be examined with the time-trade-off method, where patients are given a hypothetical scenario where the typical postoperative course for surgery is described in detail and patients are asked if an increased survival of 6 months, for example, would lead them to choose surgery and its associated recovery in order to gain this survival benefit. The anticipated increase in survival is then lessened and the same question asked again, with the process repeated until the patient no longer reports they would be willing to have surgery for the anticipated survival benefit. Such a study could provide information as to whether most patients would consider resection of the primary, even if it were proven to be associated with some definite increase in survival.
Thus, it remains unclear whether resection of the primary tumor is associated with a decrease in complications from the primary tumor or an increase in survival. Patient preferences in this area have not been well studied and are likely to play a significant role in whether surgical intervention for the primary tumor is pursued.
3 Standard Versus Extended Lymph Node Resection for Colon and Rectal Cancer
Great emphasis has been placed on ensuring appropriate oncologic resection for colon and rectal cancer, with 12 nodes in the specimen being a generally accepted standard to ensure an adequate resection and accurate staging. This standard does not take into account factors that are associated with decreased lymph node number (such as radiation, left-sided resections or variations in pathology practice), [37] or whether an increased number of lymph nodes was obtained by resecting a greater length of bowel, a longer segment of the feeding vessel, or a more complete mesocolic/mesorectal excision. Some have advocated a more aggressive resection of the lymph nodes, beyond this numerical standard, for both colon cancer and for rectal cancer. It remains unclear whether this results in better oncologic outcomes.
3.1 Colon Cancer
For colon cancer, more aggressive resection is termed complete mesocolic excision (CME). For the right colon, this entails a Kocher maneuver with mobilization of the mesenteric root up to the base of the superior mesenteric artery, including dissection of the mesentery off of the uncinate process of the pancreas and duodenum. For the left colon, this involves takedown of the splenic flexure and resection of the transverse mesocolon at the lower edge of the pancreas [38]. In both cases, close attention is paid to maintaining an intact mesocolic fascia. Some authors also resect a greater length of colon as part of this approach [39, 40]. Similar to total mesorectal excision in rectal cancer, the theory is that maintaining intact embryologic planes and ensuring complete resection of the mesentery will improve oncologic outcomes.
Results of this approach have generally shown acceptable operative times, blood loss and postoperative complications [38, 41, 42]. CME has also been demonstrated to result in a greater incidence of an intact mesocolon and a greater number of lymph nodes resected [39, 40]. Attempts have been made to determine whether this results in improved oncologic outcomes. Hohenberger et al. [38] showed that among node-negative patients, those with resection of 28 or more lymph nodes had 96.3 % cancer-related 5-year survival versus 90.7 % if less than 28 lymph nodes were resected, but a similar analysis among node-positive patients was not significant. CME was the standard practice at that institution, so the differences in survival were not associated with whether CME was attempted. Other studies have shown improved outcomes with preservation of anatomic planes [43, 44] or more extensive resection [45]. However, it remains unclear whether there is a benefit in terms of survival or local recurrence. The primary problem is that individual centers tend to pursue either CME or standard resection exclusively, and there are therefore no appropriate patients to serve as comparators. Comparing outcomes from patients operated on at different centers introduces an increased risk that any differences observed are due to factors other than the surgical approach.
Conclusive evidence demonstrating whether there is a benefit to CME would require a randomized clinical trial of CME versus more standard resection. However, the number of patients required to demonstrate this difference could be prohibitive, depending on the endpoints chosen for the study. As an example, power calculations can be estimated using the local recurrence rate (4.8 %) and cancer-specific survival rate (85.2 %) from Weber et al.’s [42] study of 1,452 patients undergoing CME who were followed for at least 5 years. Assuming an 80 % power and a significance level of 0.05, 11,136 patients would be required to demonstrate a 25 % difference in local recurrence. In contrast, 792 patients would be required to demonstrate a 10 % difference in cancer-specific survival. While this latter example may represent a feasible number of patients to recruit to such a study, the number of patients required is exquisitely sensitive to the estimated difference in cancer-specific survival, and the 10 % difference estimate may be too high. Changing the estimated difference in cancer-specific survival to 5 % rather than 10 % increases the number of patients required to 2,614.
A more feasible approach may be a pathology-based study. CME could be performed in patients, and the surgeon could delineate which areas of the specimen they believe would have been removed in a standard resection and which areas were resected only as a result of the CME. It may be best to have two surgeons come to an agreement regarding these boundaries, to prevent the surgeon from under- or overestimating the amount of tissue that would have been removed with a standard resection, as Spasojevic et al. [46] found surprising lengths of artery remaining after what were reportedly standard resections with high ligation. The two areas of the specimen could then be dissected apart and processed separately. If additional nodal metastases or tumor deposits are found frequently in the additional tissue, this would lend more credence to the argument to perform a more extensive resection. If, however, metastasis is infrequent in the additional tissue, this would make it unlikely that CME contributes to a clinically significant difference in outcomes. A similar study, looking at the location of lymph node metastases in right-sided colon cancers, found less than 1 % of lymph node metastases were located more than 10 cm from the primary tumor (Fig. 1) [47].
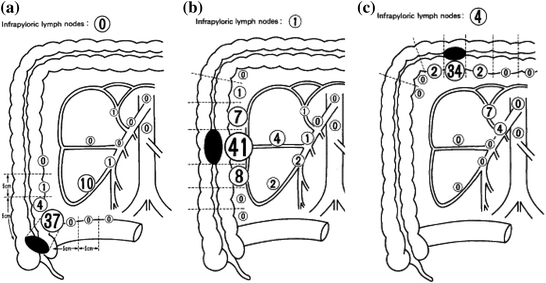

Fig. 1
Rates of lymph node metastases for cecal (a), ascending (b) and transverse colon cancers (c). From Toyota et al. [47]
3.2 Rectal Cancer
In contrast to colon cancer, in rectal cancer dissection along embryologic planes is accepted practice. However, controversy remains about whether more extensive lymph node dissection is of benefit. Particularly in Japan, a more aggressive resection is often used, including dissection of the lateral pelvic lymph nodes. This has been shown to result in increased survival [48, 49] in some studies, although other studies have shown no difference [50, 51]. A recent meta-analysis showed no difference in overall or disease-free survival (Fig. 2) [52].
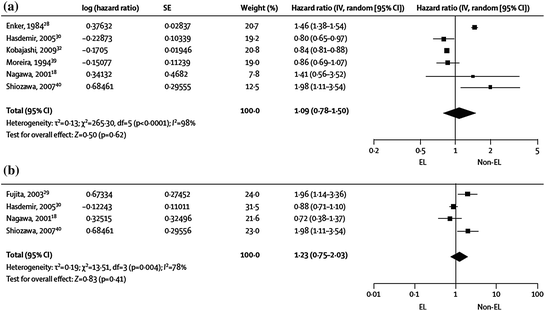

Fig. 2
Meta-analysis of 5-year overall (a) and disease-free survival (b) following extended versus non-extended lymphadenectomy for rectal cancer. Squares are point estimates of the treatment effect, with 95 % CI indicated by horizontal bars. Diamonds are the summary estimate from the pooled studies with 95 % CI. From Georgiou et al. [52]
There are a few issues that make it difficult to determine whether extended lymph node resection for rectal cancer is associated with a benefit. For the studies demonstrating a survival advantage with more aggressive lymph node resection, stage migration could be a confounding issue. More accurate staging due to a larger number of lymph nodes being resected could theoretically correctly classify some early stage III cancers that would have been erroneously classified as stage II, thus improving the survival of both stage II and stage III cancers as a whole, even if there is no actual survival benefit for extended lymph node resection. Another pertinent issue is the use of radiation. Many of these patients do not receive preoperative chemotherapy and radiation. However, in other countries where preoperative radiation for node-positive rectal cancer is more common, such as the United States, extended node dissection is not standard practice. There is some evidence that these two approaches have similar effectiveness [53]. However, it is unclear whether adding extended node dissection to radiation may further improve outcomes. Finally, the average body mass index is generally much lower in countries which practice extended lymph node resection, raising the question of whether this technique can be generalized while maintaining the same results.
Whether lateral node dissection should be undertaken is particularly important because there can be significant adverse effects. In this dissection, the pelvic nerves are often damaged or even intentionally sacrificed, and this results in a very high incidence of urinary and sexual dysfunction [50, 54, 55]. For example, Akasu et al. [54] found that with unilateral or bilateral pelvis plexus sacrifice, rates of inability to have sexual intercourse at 1 year were 55 and 100 %, respectively. Urinary symptoms were present in 100 % of male and 90 % of female patients in one study, and 1 year after surgery 44 % of men and 17 % of women still required self-catheterization [55]. Lateral node dissection has also been associated with increased operative time [50, 52, 56] and blood loss [52, 56].
Ideally, a randomized controlled trial would determine whether extended lymph node resection contributes any additional benefit among patients who have undergone preoperative chemotherapy and radiation. Indeed, this study has already been performed by Nagawa et al. [57]. They found no difference in survival between patients undergoing a standard resection versus an extended lymph node resection after preoperative chemotherapy and radiation. However, only 45 patients were enrolled in this study, severely limiting its ability to detect a difference in survival. In addition, patients with evidence of lateral pelvic or para-aortic lymph node metastasis were excluded, although it seems this staging may have been done after chemotherapy and radiation were completed.
A more informative study may be obtained by selecting only patients who have evidence of lateral pelvic or para-aortic lymph node metastasis before chemotherapy and radiation, then randomizing them to standard versus extended lymph node resection after neoadjuvant therapy. Limiting this analysis to patients who are preoperatively known to have advanced nodal metastasis achieves two ends. One, it increases the likelihood of local recurrence [49] and therefore decreases the sample size needed to demonstrate a difference related to extended node dissection, similar to the discussion of CME for colon cancer. Second, it could help resolve the ethical dilemma of exposing patients to the high risk of urinary and sexual dysfunction associated with lateral node dissection; if patients are strongly suspected to have lateral node metastasis prior to radiation these risks may be more acceptable in light of an increased risk of poor outcome. Thus, the pertinent question seems to be not whether radiation or extended lymph node resection is preferable, but if extended resection can improve upon the outcomes already obtained with preoperative chemotherapy and radiation in cases of known lateral pelvic or para-aortic lymph node metastasis, and whether the combination of radiation and extended lymph node resection would lead to prohibitively severe morbidity.
While extended lymph node resection for colon and rectal cancer seems as if it would be preferable, current data do not allow conclusions to be drawn about whether this approach has benefits that would justify any increase in operative time or complications. A variety of methods may be used to provide additional data in this area.
4 Robotic Versus Laparoscopic Resection for Colorectal Cancer
When reports of laparoscopic colon and rectal resection first appeared, the relative merits of laparoscopic and open surgery were hotly debated. Many surgeons now believe that laparoscopic resection is associated with improved postoperative recovery and similar oncologic outcomes, but even so both open and laparoscopic surgery continue to have their proponents, and only 10.5 % of elective colon resections in the United States were performed laparoscopically as recently as 2007 [58]. The appearance of robotic surgery has renewed the debate regarding the optimal approach to colorectal cancer resections.
Potential benefits of the robotic approach center around the ability to have seven degrees of freedom in movement, and the increased visibility resulting from a three-dimensional view and greater magnification (Table 4). Particularly in the pelvis, where dissection can be quite difficult due to the bony pelvis, patient obesity and a bulky tumor, these may be significant advantages. Laparoscopic resections in this area can be difficult due to the fulcrum effect of the laparoscopic instruments on the sacral promontory. The robot also stabilizes physiologic tremor. Robotic surgery has thus been hypothesized to result in a better surgical specimen, with some evidence to support this [59].
Table 4




Potential advantages of robotic colorectal surgery as compared to laparoscopic surgery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree