Reference
Surgery
Obese/T2DM
Stimulus
GLP-1
GIP
RYGBP
OB
Meal
↓
Halverson et al. [78]
RYGBP
OB
OGTT
↑
Sirinek et al. [79]
RYGBP
OB
OGTT
↓
Naslund et al. [80]
JIB
OB
Meal
↑
↑
Verdich et al. [81]
Diet
19 OB/12 lean
Meal
↑
↓
Valverde et al. [82]
BPD/VBG
OGTT
↑
Korner et al. [83]
RYGBP
OB/lean
Meal
↑
↓
Borg et al. [84]
RYGBP
OB
Meal
↑
Morinigo et al. [49]
RYGBP
OB
Meal
↑
Laferrère et al. [61]
RYGBP
OB/T2DM
OGTT
↑
↑
Jorgensen et al. [85]
RYGBP
OB/T2DM/NGT
Meal
↑
–
Jacobsen et al. [65]
RYGBP
OB
OGTT
↑
–
Romero et al. [86]
VSG/RYGBP
OB/T2DM
Meal
↑
↑
Mallipedhi et al. [87]
VSG/BPD
IGT/T2DM
OGTT
↑
↓
Plourde et al. [88]
BPD
T2DM/NGT
Meal
↑
↓
Kim et al. [64]
RYGBP
Lean T2DM
OGTT
↑
↓
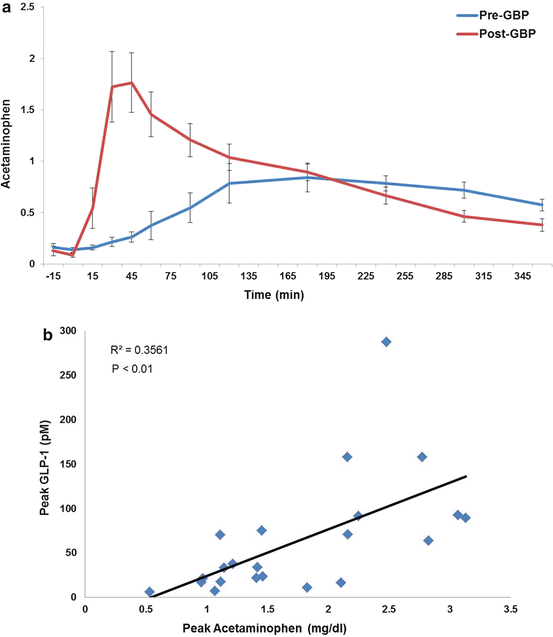
Fig. 9.1
Accelerate gastric pouch emptying after RYGBP. (a) Acetaminophen levels during a 600 kcal liquid meal given before or 1 year after RYGBP surgery; (b) Strong relationship between gastric pouch emptying and GLP-1 release during a 600 kcal meal
9.6 Lessons from Rodent Models
Although data in humans and pigs support a role for GLP-1 in controlling glucose after RYGBP, experiments with knock out (KO) animal models challenge the role of GLP-1 in the control of body weight and glucose after RYGBP or VSG. Berthoud et al. [89] showed that chronic brain infusion of exendin-9-39 into the lateral cerebral ventricle similarly increased food intake and body weight in both RYGBP and sham-operated rats, suggesting that, while contributing to the physiological control of food intake and body weight, central GLP-1 receptor signaling tone is not the critical mechanism uniquely responsible for the body weight-lowering effects of RYGBP. In a separate experiment, the same authors showed that obese GLP-1R-deficient mice lost the same amount of body weight and fat mass and maintained similarly lower body weight compared with wild-type mice after a RYGBP-like procedure [89]. GLP-1 levels are also enhanced after VSG in humans [90] and rodents [91], and are thought to be a mediator of diabetes remission after this surgery [92]. However, VSG-operated GLP-1 receptor-deficient mice respond similarly to wild-type controls in terms of body weight loss, improved glucose tolerance, food intake reduction, and altered food selection [93]. These data demonstrate that GLP-1 receptor activity is not necessary for the metabolic improvements induced by VSG or RYGBP surgery in these animal models. The relevance of these KO experiments to clinical observations is unclear.
9.7 Effect of RYGBP on the Incretins: Does It Matter for Beta Cell Function?
The main effect of the incretins is enhancement of glucose stimulated-insulin secretion. In order to single out the incretin effect from the weight loss effect of the surgery, we have used two approaches. One is to compare the effect of an oral glucose challenge to that of an isoglycemic IV glucose clamp on beta cell function, to quantify the incretin effect. Presumably, the change in beta cell response to oral glucose after RYGBP would engage neural and hormonal gut mechanisms, while the response to IV glucose would only be a function of the change in glycemia related to weight loss. The other approach is to block the effect of the endogenous incretins. Although there is no available GIP receptor inhibitor for human use, the specific GLP-1 receptor inhibitor exendin 9-39 has been used in four cross sectional [94–97] studies and one longitudinal short term [98] study in post-RYGBP patients. Exendin 9-39 administration has little to no effect prior to surgery, but completely blunts the recovery of beta cell glucose sensitivity (BCGS) , or the insulin secretin rate in response to incremental changes in blood glucose during a glucose challenge, observed 1 week and 3 months after RYGBP [98]. The administration of Exendin 9-39 worsens postprandial glucose tolerance, although only minimally [97]. Exendin 9-39 suppresses insulin secretion in response to a meal by 50 % [94, 97] and corrects the profound reactive hypoglycemia in patients with severe neuroglycopenia [94]. So clearly the exaggerated GLP-1 response to ingestion of food or glucose plays a key role in postprandial insulin secretion and glycemic control after RYGBP. To assess beta cell function, we measured BCGS and the disposition index (DI), i.e. the relationship between insulin secretion and insulin sensitivity. Both measures were calculated using data from an oral glucose load and from a matched isoglycemic IV glucose load, collected on separate days, in patients with T2DM and severe obesity, before and at 1 month, then yearly for 3 years after RYGBP surgery. Prior to surgery, BCGS after either an oral or IV isoglycemic glucose challenge, was, as expected, significantly impaired in patients with T2DM compared to lean controls, and to obese controls with normal glucose tolerance (NGT), matched for BMI. After RYGBP, all patients were in diabetes remission (HbA1C < 6.5 %, fasting glucose <126 mg/dl, on no diabetes medications). The BCGS and DI measured using parameters derived from the oral glucose test improved rapidly at 1 month and normalized to the levels of the lean and the obese NGT controls at 1 year. However, BCGS and DI measured after IV glucose administration improved only minimally and remained much impaired compared to that of the lean and obese NGT non-operated controls [99]. This experiment highlights the role of the incretins and other gut-mediated factors in the amelioration of beta cell response to oral nutrients after RYGBP. It also clearly shows a persistent beta cell defect that cannot be rescued with an IV glucose challenge, 3 years after the surgery, even in persons who are in clinical diabetes remission. In humans, there is no evidence to date for a full recovery of beta cell function to IV stimuli in patients in diabetes remission [99].
In order to distinguish between a caloric restriction/weight loss effect and an effect independent of weight loss, we compared individuals studied before and after RYGBP to individuals studied before and after an equivalent 10 % weight loss by caloric restriction with or without AGB. BCGS and DI after IV glucose stimulus improved significantly and similarly after the two modes of weight loss. After the oral glucose challenge, beta cell function improved significantly more after RYGBP than after diet. Results from this experiment underscore the importance of the engagement of the gut and the incretin effect, rather than weight loss, in the metabolic response to nutrient stimulation after RYGBP [100].
9.8 Conclusions
Bariatric surgery and its associated weight loss result in diabetes remission. The effect of the incretins hormones on postprandial insulin secretion and glucose control is amplified after RYGBP, as a result of the accelerated passage of nutrients. The enhanced incretin effect rescues beta cell function, independent of weight loss. This effect, observed only during meals, may not play a predominant part in diabetes remission after RYGBP.
References
1.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8.
2.
Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wagenknecht LE, Wing RR, Look ARG. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126(3):236–42. doi:10.1016/j.amjmed.2012.10.010. 242e.1–2.PubMedCentralCrossRefPubMed
3.
Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM, Longitudinal Assessment of Bariatric Surgery Consortium. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–25. doi:10.1001/jama.2013.280928.PubMedCentralPubMed
4.
Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi:10.1001/jama.2011.1914.CrossRefPubMed
5.
Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. discussion 350–332.PubMedCentralCrossRefPubMed
6.
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76. doi:10.1056/NEJMoa1200225.PubMedCentralCrossRefPubMed
7.
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. doi:10.1056/NEJMoa1200111.CrossRefPubMed
8.
Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146(2):143–8. doi:10.1001/archsurg.2010.326.CrossRefPubMed
9.
Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, Ahmed L, Vella A, Chuang LM, Bessler M, Sarr MG, Swain JM, Laqua P, Jensen MD, Bantle JP. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9. doi:10.1001/jama.2013.5835.PubMedCentralCrossRefPubMed
10.
Lo Menzo E, Szomstein S, Rosenthal RJ. Changing trends in bariatric surgery. Scand J Surg. 2014. doi:10.1177/1457496914552344.PubMed
11.
Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, Pothier CE, Brethauer S, Nissen S, Gupta M, Kirwan JP, Schauer PR. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36(8):2175–82. doi:10.2337/dc12-1596.PubMedCentralCrossRefPubMed
12.
Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. doi:10.1097/01.sla.0000089851.41115.1b. discussion 484–465.PubMedCentralPubMed
13.
Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–23. doi:10.1001/jama.299.3.316.CrossRefPubMed
14.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi:10.1001/jama.292.14.1724.CrossRefPubMed
15.
Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, O’Connor PJ, Theis MK, Campos GM, McCulloch D, Selby J. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23(1):93–102. doi:10.1007/s11695-012-0802-1.PubMedCentralCrossRefPubMed
16.
Guldstrand M, Ahren B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab. 2003;284(3):E557–65. doi:10.1152/ajpendo.00325.2002.CrossRefPubMed
17.
Villareal DT, Banks MR, Patterson BW, Polonsky KS, Klein S. Weight loss therapy improves pancreatic endocrine function in obese older adults. Obesity. 2008;16(6):1349–54. doi:10.1038/oby.2008.226.PubMedCentralCrossRefPubMed
18.
Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23. doi:10.1007/s11605-009-1060-y.PubMedCentralCrossRefPubMed
19.
Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, Patterson BW, Klein S. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122(12):4667–74. doi:10.1172/JCI64895.PubMedCentralCrossRefPubMed
20.
Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–85. doi:10.1210/jc.2007-2851.PubMedCentralCrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree