Radial scope
Convex scope
Advantage
• Scanning range is 360°
• Pancreas is easily seen as a longitudinal and continuous image
• Histological diagnosis is possible
• Junction between the pancreatic head and body can be seen from the stomach
Disadvantage
• Histological diagnosis is impossible
• Operator dependent
• Scanning range is 180°
• Images of the body and tail of the pancreas become cross-sectional images
1.2 EUS (Fig. 1.1)
The EUS equipment includes probes with different imaging methods: radial probes allow 360° imaging perpendicular to the long axis, and convex probes allow imaging along a plane parallel to the long axis of the instrument. The former only allows diagnostic imaging, whereas the latter was developed for fine-needle aspiration (FNA) [3, 4]. EUS uses high ultrasound frequencies, with imaging from the stomach or duodenum providing high resolution, real-time images of the pancreas. This modality therefore plays an important role in evaluating pancreatic diseases.


Fig. 1.1
Scheme of radial EUS and convex EUS. Radial EUS has 360° imaging perpendicular to the long axis. MPD is depicted longitudinally in pancreas body. Convex EUS has imaging along a plane parallel to the long axis of the instrument. MPD is depicted short axis view in pancreas body
1.3 Early Diagnosis of PC Using EUS
MDCT evaluation of patients with suspected PC is the standard preoperative assessment at most medical institutions. This is because MDCT has good spatial and temporal resolution with wide anatomical coverage, and thus permits both comprehensive local and distant disease assessment during a single session [5, 6].
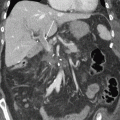
Among cross-sectional imaging modalities, the performance of MDCT is optimal for evaluating vascular involvement, which is the most important predictor of tumor resectability [7–9]. However, about 10% of PCs are iso-attenuating relative to the background pancreatic parenchyma (Fig. 1.2) [10]. CT enhancement of the PC and of pancreatic parenchyma surrounding a tumor is correlated with the degree of fibrosis. Contrast material is retained in PC with a predominant fibrous component. A similar degree of fibrosis in a tumor and surrounding pancreatic parenchyma might lead to overlapping enhancement on MDCT that could prevent the detection of PC, especially when tumors are ≤2 cm [11–13].
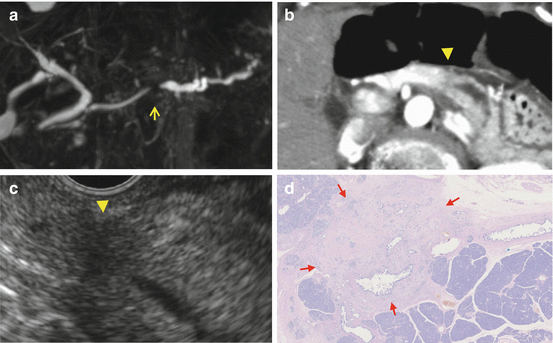

Fig. 1.2
Case: a 8 mm pancreatic cancer with invasion. MRCP (a) showed short duct stenosis in pancreatic body (arrow). Contrast-enhanced CT (b) could not detect the mass in pancreatic body (arrow ahead) though main pancreatic duct (MPD) was dilated and disrupted in the body. (c) EUS could detect the low echoic mass with unclear margin. (d) Microscopic findings revealed an 8 mm tumor with invasion accompanied with 20 mm fibrosis
On the other hand, PC appears on EUS images as heterogeneous hypoechoic masses with irregular margins, which allows very high sensitivity for detecting PC [14, 15].
It is considered one of the most accurate means of detecting pancreatic focal lesions, especially when tumors are ≤2 cm [16–19].
Recent reports indicate that EUS can detect tumors <10 mm [20–22]. The sensitivity of EUS for detection of 25 small PC with size <10 mm was 84%, among eight Japanese high-volume centers [23]. Therefore, all patients with obstructive jaundice or unexplained pancreatic duct dilation, in whom CT or MRI do not definitively identify pancreatic lesions should be assessed using EUS [24].
However, EUS can miss a true pancreatic mass in patients with chronic pancreatitis, a diffusely infiltrating carcinoma, a prominent ventral/dorsal split, or a recent episode (<4 weeks) of acute pancreatitis [25]. The potential for suboptimal visualization of the pancreatic gland for detection of PC by EUS and other imaging modalities should be acknowledged in the setting of acute or chronic pancreatitis. Acoustic shadowing caused by an indwelling biliary or pancreatic stents, or pancreatic stones can also interfere with the visualization of small pancreatic masses.
1.4 Pancreatic Intraepithelial Neoplasia (PanIN)
PC develops through stepwise progression from precursor lesions comprising pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN). Among these, PanIN is the most common precursor of PC [26]. PanIN are noninvasive epithelial proliferations within smaller pancreatic ducts (<0.5 mm) that can be flat or papillary and classified into low (PanIN-1), intermediate (PanIN-2), and high (PanIN-3) grades according to the degree of architectural and cellular atypia [26]. Based on mutations associated with each grade, normal ductal epithelium seems to progress through low-grade PanIN, high-grade PanIN, localized adenocarcinoma, and metastatic adenocarcinoma in that order. Detection of high-grade PanIN-3 would provide an optimal opportunity to reduce mortality from PC. It has been believed that PanIN cannot be reliably visualized using clinical imaging [27] as they typically arise in the small-caliber pancreatic ducts [26].
However, it has been recently suggested that PanIN is associated with localized parenchymal changes that may be detected by EUS [28, 29]. These parenchymal changes are characterized by acinar cell loss, proliferation of small ductular structures, and fibrosis referred to as lobulocentric atrophy (LCA) [30]. Localized fibrosis and/or LCA has been pathologically identified in parenchyma around PanIN-3 [21, 25, 28, 29, 31, 32]. A slightly low echoic lesion on EUS images might suggest localized fibrosis around PanIN-3 [28]. Maire et al. [29] reported that EUS changes corresponded to PanIN lesions in 83%. EUS also detected 69% of patients with PanIN lesions and 57% of those with PanIN3 lesions. However, EUS findings for PanIN lesion were not uniformed. For instance, Maire et al. [29] defined EUS findings of PanIN lesion were microcysts or hyper-echogenic foci resulting in a heterogeneous pattern. On the other hand, Hanada et al. [28] reported slightly low echoic lesion on EUS images were the findings of PanIN. However, it should be noted that these abnormalities on EUS are not specific to PanIN or early PC, and conversely, PanIN may well occur in the absence of LCA [30, 33]. Further studies are warranted to confirm these findings.
1.5 Surveillance of High-Risk Individuals
Familial pancreatic cancer (FPC) kindreds are defined as families with two or more first-degree relatives (FDR) affected with PC, in the absence of other cancers or familial diseases. Klein et al. found that the risk of developing PC was 4.5- vs. 32-fold depending on whether one or at least three FDR were affected, respectively [34, 35].
A multicenter prospective cohort study (CAPS 3) implemented by Canto et al. [36] included 216 high-risk individuals (HRI) (Peutz-Jeghers syndrome, n = 2; familial breast-ovarian cancer with at least one affected first- or second-degree relative with PC, n = 19; relatives of patients with FPC with at least two FDR, n = 195). All persons were evaluated by CT, MRI, and EUS, and 92 (42%) of 216 had at least one pancreatic mass (84 cystic and 3 solid) or a dilated pancreatic duct (n = 5) according to the findings of at least one of the imaging modalities. The prevalence of these lesions increased with age of the screened persons. Pancreatic abnormalities were detected by CT, MRI, and EUS in 11%, 33.3%, and 42.6% of the patients, respectively. Among the pancreatic lesions, 82 were IPMN, and three were neuroendocrine tumors. Five patients who were surgically treated had high-grade dysplasia in IPMN <3 cm and multiple intraepithelial neoplasms. Canto et al. concluded that screening asymptomatic HRI could detect curable noninvasive high-grade and multiple cystic lesions. Both EUS and MRI were more effective diagnostic screens for HRI than CT [37]. These findings showed that screening of high-risk families can detect early precancerous changes in the pancreas [35].
1.6 New Screening Modality Comprising Contrast EUS and Elastography
Conventional EUS sometimes cannot detect pancreatic tumors in patients with chronic pancreatitis, diffusely infiltrating carcinoma, or a recent episode of acute pancreatitis [25]. Contrast-enhanced (CH)-EUS and EUS elastography might help to improve the diagnostic accuracy of EUS.
Parenchymal perfusion and the pancreatic microvasculature can be visualized without artifacts by CH-EUS [38], and it is useful in the differential diagnosis of PC, especially small tumors [39, 40]. Fusaroli et al. [41] reported that pancreatic tumor visualization by CH-EUS is better than that of conventional EUS. A recent meta-analysis of 1139 patients found that the sensitivity and specificity of CE-EUS for a differential diagnosis of PC were 94% and 89%, respectively [39]. That study found that hypo-enhancing lesions on CE-EUS images were a sensitive and accurate predictor of PC.
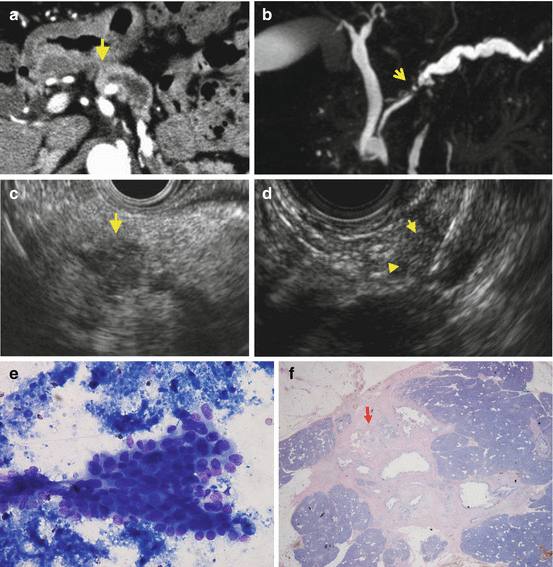

Fig. 1.3
Case: a 3 mm pancreatic cancer with invasion. Contrast-enhanced CT (a) could not detect the mass in pancreatic head (arrow ahead) although main pancreatic duct (MPD) was dilated. MRCP (b) showed short duct stenosis in pancreatic body (arrow). (c) EUS showed an 7 mm low echoic mass (arrow). (d) EUS-FNA was performed from the 7 mm low echoic mass using 22G FNA needle. (e) Cytology with Papanicolaou stain showed atypical cells consistent with adenocarcinoma. (f) Macroscopic findings revealed pancreatic adenocarcinoma with invasive components of 3 mm (arrow) with 8 mm surrounding fibrosis
Because CH-EUS is more sensitive, it can be used to identify targets of EUS-FNA [41–43] and might also help to avoid puncturing necrotic and inflammatory areas of malignant masses or hard and scirrhous areas of inflammatory masses, thus reducing the need for repeated FNA assessments.
Another emerging technology is EUS elastography, which provides real-time visualization of tissue stiffness. It is based on the premise that compression causes less strain in hard, rather than in soft tissues [44]. The results of recent investigations using EUS elastography for diagnosing pancreatic focal lesions are promising [45–47]. As malignant lesions are generally harder than normal adjacent tissue, measuring strain might help to classify pancreatic masses. Two meta-analyses recently found high pooled sensitivity (95–97%) and low pooled specificity (67–76%), for a differential diagnosis of solid pancreatic masses [48, 49].
1.7 Early Diagnosis of PC Using EUS-FNA (Fig. 1.3)
Although EUS has high overall sensitivity, differentiating PC from other solid lesions based only on endosonographic features remains challenging. Specimens for histopathological diagnosis can be collected using EUS-guided FNA. Since its introduction in the early 1990s, EUS-FNA has emerged as a safe and accurate means of tissue diagnosis in patients with pancreaticobiliary disorders, particularly confirmed PC. The sensitivity and specificity of EUS-FNA for diagnosing pancreatic masses is 80–95% and 75–100%, respectively [51–55].
Uehara et al. [56] recently reported that EUS-FNA was 96% accurate for identifying pancreatic masses <10 mm in 23 patients. Thus, EUS-FNA is useful for confirming pancreatic tumors <10 mm.
References
1.
Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree