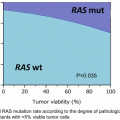
Risk factors
Relative risk (-fold)
Age (≥65 years)
>4.0
Genetic factors: BRCA-1 and BRCA-2
>4.0
Lobular carcinoma in situ (LCIS)
7.0–11.0
Family history
One first-degree relative
2.0
Two first-degree relatives
3.0
Personal history of breast cancer
Early onset (<40 years)
>4.0
≥40 years
2.1–4.0
Dense breasts
>4.0
Proliferative lesions without atypia
1.5–2.0
Ductal hyperplasia
Fibroadenoma
Sclerosing adenosis
Radial scar
Papillomatosis
Proliferative lesions with atypia
3.5–4.0
Atypical ductal hyperplasia (ADH)
Atypical lobular hyperplasia (ALH)
Previous chest radiation (especially for Hodgkin or non-Hodgkin lymphoma)
2.1–4.0
Early menarche (before age 12) or late menopause (>55 years)
1.1–2.0
Ashkenazi (Eastern European) Jewish heritage
1.1–2.0
Diethylstilbestrol (DES) exposure
1.1–2.0
Nulliparity, first child after age 30
1.1–2.0
Birth control pill, hormone replacement therapy
1.1–2.0
Alcohol consumption
1.1–2.0
Obesity
1.1–2.0
Inactivity
1.1–2.0
History of endometrium, ovary, or colon cancer
1.1–2.0
Gail Model of Risk Assessment
Given a set of risk factors, what is the probability that a woman will develop breast cancer during the next 5 years and by age 90 years? While there are several models such as Gail [5], Claus [6], or Tyrer-Cuzick [7] that estimate breast cancer risk, the Gail model is the more widely known. The most updated version of the Gail model has been implemented into a variety of formats, one of which is the Breast Cancer Risk Assessment Tool (BCRAT); BCRAT is available on the National Cancer Institute (NCI) website (http://cancer.gov/bcrisktool). The BCRAT incorporates information such as patient’s age, age at menarche, age at first live birth, number of 1st degree relatives with breast cancer, number of prior breast biopsies, presence of atypia on biopsy, and race/ethnicity. Such a model informs the patient and her health-care providers of her risk and allows them an opportunity to select appropriate management options to reduce such a risk.
Histologic Subtypes
The female adult breast is arranged into 15–20 lobes, with each lobe made up of many smaller lobules. The lobules are all linked by ducts, which terminates in the nipple. Adipose tissue fills the spaces between lobules and ducts (Fig. 4.1). Each breast contains a rich network of blood vessels and lymphatics, the latter drains into the axilla, supraclavicular region, internal mammary lymph nodes, as well as other parts of the body (Fig. 4.2).
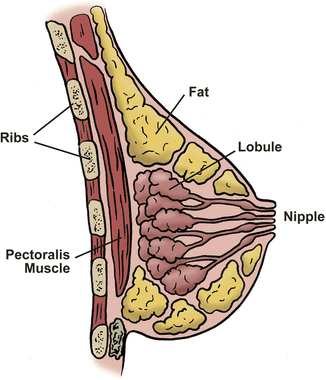
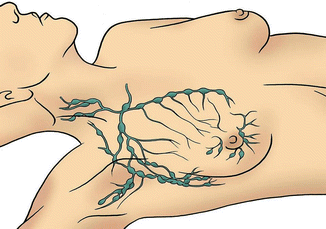

Fig. 4.1
Female breast anatomy: the ductal-lobule system is comprised of numerous lobules with acini. Each lobule empties into a terminal duct, which empties into a segmental duct, and finally into the collecting ducts. There are about 15–20 collecting ducts that converge under the areola (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 4.2
Lymphatic system of the breast: lymphatic drainage begins from the breast lobules and flow into the axillary lymph nodes, internal mammary lymph nodes, retromammary lymph nodes, and infraclavicular and supraclavicular lymph nodes. The axillary lymph nodes receive approximately 75 % of the lymph drained from the breast. Lymphatics may also reach the sheath of the rectus abdominis, the subperitoneal and subhepatic plexuses, and the contralateral breast (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Breast cancer encompasses a variety of histologies including ductal, lobular, medullary, tubular, and metaplastic. Of these, invasive ductal carcinoma (IDC) is the most common, accounting for about 80 % of the cases [8, 9] (Fig. 4.3v). Invasive lobular carcinoma (ILC) is the second most common, accounting for about 8–15 % of all invasive breast cancer [8, 10]. ILC typically arranges itself in a linear arrangement (single-file pattern), has a tendency to grow around ducts and lobules in a circumferential manner, and is associated with desmoplastic stromal reaction (Fig. 4.3b). Compared to IDC, ILC tends to be well differentiated; has a lower rate of HER-2 overexpression [11], higher rate of hormone receptor positivity [10], and higher incidence of bilaterality, multifocality, and multicentricity [12, 13]; and is more likely to harbor micrometastatic disease [14]. Other histologic types that are associated with a good prognosis are medullary, mucinous, tubular, cribriform, and adenoid cystic carcinoma of the breast, while micropapillary carcinoma and angiosarcoma are the more aggressive subtypes (Fig. 4.3c–f). Regardless of the subtypes, treatment is the same for all because current treatment guidelines and decision strategies are not dependent on subtypes.
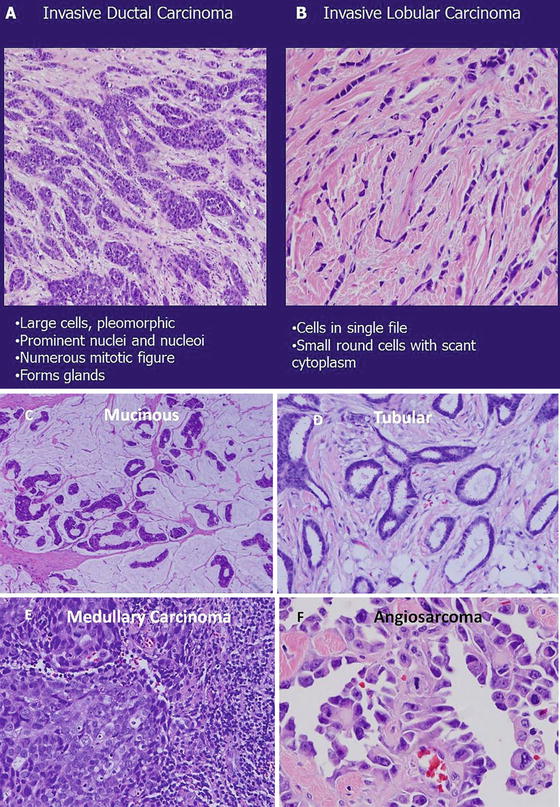

Fig. 4.3
Hematoxylin and eosin (H&E) sections of invasive ductal carcinoma (a) and invasive lobular carcinoma (b). Mucinous carcinoma is characterized by accumulation of abundant extracellular mucin around invasive tumor acini, nests, and trabeculae (c; low power, 40×). Tubular carcinoma (d; high power 100×): the glands reveal angular contours and are composed of single layer of neoplasm epithelial cells. The nuclear pleomorphism is lower grade. Stroma fibrosis and elastosis are associated with infiltrative invasion. Medullary carcinoma (e; high power 100×): this tumor is characterized by nodular expansion and peripheral lymphocytic infiltration. The tumor grows as syncytial sheets surrounded by a diffuse lymphocytic reaction. The tumor cells are poorly differentiated with high nuclear pleomorphism and mitotic rate. Angiosarcoma (f): this tumor is high grade with dilated vascular spaces that are dissecting and splitting tissue planes. The vascular spaces are lined and filled with highly pleomorphic malignant cells with easily identified mitosis (a–f: Courtesy of Quyen D. Chu, MD, MBA, FACS)
Evaluation and Management of Early Breast Cancer
Once a diagnosis of breast has been made, the clinician should ask the following important questions: am I dealing with (1) an operable or early breast cancer (EBC), (2) locally advanced breast cancer (LABC) or inflammatory breast cancer (IBC), or (3) metastatic breast cancer? This is important because the answer will help guide her/him in selecting the most appropriate treatment option.
The answer to be above question is based on the clinician’s initial assessment of the patient’s clinical presentation. This is referred to as the clinical stage, which is different from the pathologic stage; the latter is based on the final pathology after the definitive operation. There are occasions whereby the clinician’s initial impression may be discordant with the pathologic stage (i.e., clinical impression that the patient has an EBC, but in actuality, she has advanced stage breast cancer). Regardless, the initial treatment of the patient is still predicated on the initial clinical examination. An example is a woman who presents with a breast mass that measures approximately 2.5 cm (T2) and without evidence of axillary lymphadenopathy on clinical examination (N0). Her clinical stage at this point is Stage 2 (T2N0). After undergoing definitive surgery, the final pathology demonstrated a 2.5 cm cancer (T2) with 5 out of 15 lymph nodes involved with metastatic tumor (N2). Therefore, her pathologic stage is Stage 3 (T2N2).
To determine the clinical stage, the size of the tumor and the status of the axilla should be documented. For asymptomatic patients with EBC, no further metastatic work-up is necessary. However, respiratory and neurologic complaints, bone pain, etc. necessitate a metastatic work-up. The American Joint Committee on Cancer (AJCC) TNM staging system, 7th edition, is used to stage patients with EBC [15] (Table 4.2).
Table 4.2
American Joint Committee on Cancer (AJCC) TNM Staging for Breast Cancer (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
Tis (DCIS) | Ductal carcinoma in situ |
Tis (LCIS) | Lobular carcinoma in situ |
TIS (Paget’s) | Paget’s disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget’s disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget’s disease should still be noted |
T1 | Tumor ≤ 20 mm in greatest dimension |
T1mi | Tumor ≤ 1 mm in greatest dimension |
T1a | Tumor > 1 mm but < 5 mm in greatest dimension |
T1b | Tumor > 5 mm but ≤ 10 mm in greatest dimension |
T1c | Tumor > 10 mm but < 20 mm in greatest dimension |
T2 | Tumor > 20 mm but < 50 mm in greatest dimension |
T3 | Tumor > 50 mm in greatest dimension |
T4 | Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules). Note: invasion of the dermis alone does not qualify as T4 |
T4a | Extension to the chest wall, not including only pectoralis muscle adherence/invasion |
T4b | Ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma |
T4c | Both T4a and T4b |
T4d | Inflammatory carcinoma (see “Rules for Classification”) |
Distant metastases (M) | |
|---|---|
MO | No clinical or radiographic evidence of distant metastases |
cMO(i+) | No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells in circulating blood, bone marrow, or other nonregional nodal tissue that are no larger than 0.2 mm in a patient without symptoms or signs of metastases |
M1 | Distant detectable metastases as determined by classic clinical and radiographic means and/or histologically proven larger than 0.2 mm |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage IA | T1* | N0 | M0 |
Stage IB | T0 | N1mi | M0 |
T1* | N1mi | M0 | |
Stage IIA | T0 | N1** | M0 |
T1* | N1** | M0 | |
T2 | N0 | M0 | |
Stage IIB | T2 | N1 | M0 |
T3 | N0 | M0 | |
Stage IIIA | T0 | N2 | M0 |
T1* | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N1 | M0 | |
T3 | N2 | M0 | |
Stage IIIB | T4 | N0 | M0 |
T4 | N1 | M0 | |
T4 | N2 | M0 | |
Stage IIIC | Any T | N3 | M0 |
Stage IV | Any T | Any N | M1 |
Notes | |||
*T1 includes T1mi | |||
**T0 and T1 tumors with nodal micrometastases only are excluded from Stage IIA and are classified Stage IB | |||
*M0 includes M0(i+) | |||
*The designation pM0 is not valid; any M0 should be clinical | |||
*If a patient presents with M1 prior to neoadjuvant systemic therapy, the stage is considered Stage IV and remain Stage IV regardless of response to neoadjuvant therapy | |||
Stage designation may be changed if postsurgical imaging studies reveal the presence of distant metastases, provided that the studies are carried out within 4 months of diagnosis in the absence of disease progression and provided that the patient has not received neoadjuvant therapy | |||
*Postneoadjuvant therapy is designated with “yc” or “yp” prefix. Of note, no stage group is assigned if there is a complete pathologic response (CR) to neoadjuvant therapy, for example, ypT0ypN0cM0 | |||
Regional lymph nodes (N) | |
|---|---|
Clinical | |
NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
N0 | No regional lymph node metastases |
N1 | Metastases to movable ipsilateral level I, II axillary lymph node(s) |
N2 | Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted or in clinically detected* ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases |
N2A | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures |
N2B | Metastases only in clinically detected* ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastases |
N3 | Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement or in clinically detected*ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
N3A | Metastases in ipsilateral infraclavicular lymph node(s) |
N3B | Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
N3C | Metastases in ipsilateral supraclavicular lymph node(s) |
Notes | |
*“Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis based on fine-needle aspiration biopsy with cytologic examination. Confirmation of clinically detected metastatic disease by fine-needle aspiration without excision biopsy is designated with an (f) suffix, for example, cN3a(f). Excisional biopsy of a lymph node or biopsy of a sentinel node, in the absence of assignment of a pT, is classified as a clinical N, for example, cN1. Information regarding the confirmation of the nodal status will be designated in site-specific factors as clinical, fine-needle aspiration, core biopsy, or sentinel lymph node biopsy. Pathologic classification (pN) is used for excision or sentinel lymph node biopsy only in conjunction with a pathologic T assignment | |
Pathologic (PN) | |
|---|---|
pNX | Regional lymph nodes cannot be assessed (e.g., previously removed or not removed for pathologic study) |
pN0 | No regional lymph node metastasis identified histologically |
Note: isolated tumor cell clusters (ITC) are defined as small clusters of cells not greater than 0.2 mm, or single tumor cells, or a cluster of fewer than 200 cells in a single histologic cross section. ITCs may be detected by routine histology or by immunohistochemical (IHC) methods. Nodes containing only ITCs are excluded from the total positive node count for purposes of N classification but should be included in the total number of nodes evaluated | |
pN0(i-) | No regional lymph node metastases histologically, negative IHC |
pN0(i+) | Malignant cells in regional lymph node(s) no greater than 0.2 mm (detected by H&E or IHC including ITC) |
pN0(mol-) | No regional lymph node metastases histologically, negative molecular findings (RT-PCR) |
pN0(mol+) | Positive molecular findings (TR-PCR)**, but no regional lymph node metastases detected by histology or IHC |
pN1 | Micrometastases or metastases in 1–3 axillary lymph nodes and/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detected*** |
pN1mi | Micrometastases (greater than 0.2 mm and/or more than 200 cells, but none greater than 2.0 mm) |
pN1a | Metastases in 1–3 axillary lymph nodes, at least one metastasis greater than 2.0 mm |
pN1b | Metastases in internal mammary nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected*** |
pN1c | Metastases in 1–3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected*** |
pN2 | Metastases in 4–9 axillary lymph nodes or in clinically detected****internal mammary lymph nodes in the absence of axillary lymph node metastases |
pN2a | Metastases in 4–9 axillary lymph nodes (at least one tumor deposit greater than 2.0 mm) |
pN2b | Metastases in clinically detected**** internal mammary lymph nodes in the absence of axillary node metastases |
pN3 | Metastases in 10 or more axillary lymph nodes or in infraclavicular (level III axillary) lymph nodes or in clinically detected**** ipsilateral internal mammary lymph nodes in the presence of one or more positive level I, II axillary lymph nodes or in more than three axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected*** or in ipsilateral supraclavicular lymph nodes |
pN3a | Metastases in 10 or more axillary lymph nodes (at least one tumor deposit greater than 2.0 mm) or metastases to the infraclavicular (level III axillary lymph) nodes |
pN3b | Metastases in clinically detected**** ipsilateral internal mammary lymph nodes in the presence of one or more positive axillary lymph nodes or in more than three axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected*** |
pN3c | Metastases in ipsilateral supraclavicular lymph nodes |
Notes | |
*Classification is based on axillary lymph node dissection with or without sentinel lymph node biopsy. Classification based solely on sentinel lymph node biopsy without subsequent axillary lymph node dissection is designated (sn) for “sentinel node,” for example, pN0(sn) | |
**Rt-PCR: reverse transcriptase/polymerase chain reaction | |
***“Not clinically detected” is defined as not detected by imaging studies (excluding lymphoscintigraphy) or not detected by clinical examination | |
****“Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis based on fine-needle aspiration biopsy with cytologic examination | |
In treating patients with EBC, the clinician needs to address the following: (1) local control (the primary breast tumor and the at-risk ipsilateral breast), (2) regional control (axillary lymph nodes), and (3) distant control (systemic disease). In general, the surgeon, along with her/his radiation oncologist, is responsible for the first two considerations, while her/his medical oncology colleague is responsible for the third.
Patients often present with an abnormal mammogram or breast ultrasound, with or without a palpable mass. In addition, complaints of breast pain, nipple discharge, or a palpable breast mass may also be the reasons why a patient would seek medical counseling. The initial evaluation consists of a complete history and physical examination, focusing mainly on the risk factors for breast cancer (see above), including a gynecologic history, and thoroughly examining the breast.
If the patient has no imaging, a mammogram or an ultrasound should be ordered. The most recent imaging studies should be compared with prior studies. A thorough review of the patient’s mammogram and/or breast ultrasound should be done with a radiologist. Breast ultrasound is useful for younger women (<40 years old) who tend to have denser breast tissue than older women. In some situations, both a mammogram and ultrasound might be necessary to complete the evaluation.
Classic mammographic abnormalities that are highly suggestive of a malignancy include an abnormality with irregular or spiculated borders, architectural distortion, or suspicious calcifications (linear, branching, ductally oriented, pleomorphic, clustered) (Fig. 4.4). For ultrasound, the following features are worrisome for malignancy: hypoechoic, posterior shadowing, irregular borders, nonparallel orientation, and asymmetry (Fig. 4.5).
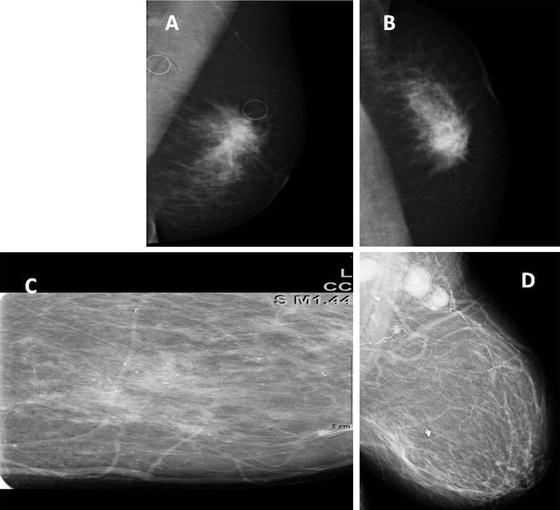
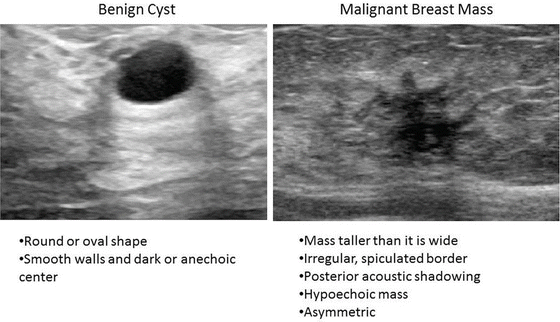

Fig. 4.4
Mammogram images (a) and (b) are views of an obscured mass in the left breast which on compression images and ultrasound was given BI-RADS 5, and pathology demonstrated an invasive ductal carcinoma. Image (c) depicts pleomorphic calcifications in a linear segmental distribution which is typical of DCIS. In this example, DCIS has evidence of cancer that has infiltrated the axillary lymph nodes (d) (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 4.5
Abnormal ultrasound (B): features that are worrisome for malignancy include an asymmetric, hypoechoic mass with irregular margins and posterior shadowing (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The American College of Radiology (ACR) has developed a standardized system of describing mammographic and ultrasound readings. The system, the Breast Imaging Reporting and Data System (BI-RADS), groups the findings into categories numbering from 0 through 6. In general, BI-RADS 0 requires additional imaging, BI-RADS 1 and 2 can be observed with annual mammogram, BI-RADS 3 requires a repeat mammogram 6 months and thereafter until the finding is known to be stable (usually at least 2 years), BI-RADS 4 and 5 require a biopsy, and BI-RADS 6 is a biopsy-proven cancer (a mammogram usually obtained after a known cancer by a previous biopsy; the mammogram, in such a case, may be used to see how sensitive the cancer is to systemic therapy) (Table 4.3). Of note, mammograms have a 10 % false-negative rate. Thus, clinical suspicion should prompt the clinician to pursue a more intense investigation despite a negative mammographic reading. On occasions, there may be discordance between the BI-RADS assignments for the mammogram and ultrasound (i.e., BI-RADS 3 for mammogram and BI-RADS 4 for ultrasound). In such a situation, we recommend to err on the conservative side and proceed with the work-up based on the highest BI-RADS reading.
Table 4.3
Breast imaging reporting and data system (BI-RADS)
BI-RADS readings | Implications |
|---|---|
0 | Additional imaging evaluation and/or comparison to prior mammograms is needed |
1 | Negative. No significant abnormality to report. Annual screening mammogram is recommended as deemed appropriate |
2 | Benign (noncancerous) finding. Annual screening mammogram is recommended as deemed appropriate |
3 | Probably benign finding. Follow-up with repeat imaging in 6 months and regularly thereafter until the finding is known to be stable (usually at least 2 years) |
4 | Suspicious abnormality. Biopsy should be considered |
5 | Highly suggestive of malignancy (95 % chance of cancer). Biopsy should be considered |
6 | Known biopsy. Proven malignancy |
A brief mentioned of screening MRI of the breast is warranted. There are no randomized trials available to determine whether screening MRI reduces mortality from breast cancer. However, screening MRI is thought to be cost effective for women between ages 30 and 60 years who either carry a BRCA mutation or have a 50 % chance of carrying such a mutation [17]. The estimated cost per quality-adjusted life-year (QALY) gained in this group is approximately $55,420–$130,625 (i.e., it costs this much per additional life-year per person). Although there is no absolute QALY threshold, in the United States, it is thought to be around $50,000–$100,000 per QALY gained, while in the United Kingdom, it is approximated to be around $30,000–$45,000 [18].
The American Cancer Society (ACS), endorsed by ACR and Society of Breast Imaging (SBI) [19], recommends annual MRI screening, along with mammography, for women at high lifetime risk (≥20 %) of developing breast cancer, beginning at age 30. The lifetime risk can be calculated using the already mentioned risk estimation models, although ACS does not recommend using the Gail model because it does not incorporate family history of ovarian or breast cancer in 2nd-degree relatives [20]; high lifetime risk includes patients with known BRCA1 or BRCA2 gene mutation or who have a 1st-degree relative with a BRCA1 or BRCA2 gene mutation and history of radiation to the chest at the ages between 10 and 30 years and patients with Li-Fraumeni syndrome or Cowden syndrome or who have a 1st-degree relative with one of these syndromes [21]. For those who are at a moderate risk of developing breast cancer (15–20 % lifetime risk), it is recommended that they discuss screening MRIs with their physicians. Moderate risk group includes those who have a personal history of breast cancer, DCIS, LCIS, ADH, atypical lobular hyperplasia, or those with extremely dense breasts or unevenly dense breasts seen on mammograms. Low-risk group includes those whose lifetime risk of breast cancer is less than 15 %; routine screening MRI is not recommended [22]. Screening MRIs should be done in a facility that also has the capability of performing an MRI-guided breast biopsy (Table 4.4). It must be stressed that MRI is not meant to replace mammography and that the guidelines are not intended to replace sound clinical judgment or considered standard of care.
Table 4.4
Recommendations for breast MRI screening
Characteristics | Time to begin screening (years) |
|---|---|
Proven BRCA mutation carriers | Annually by age 30 but not before age 25 |
Annual MRI beginning at age 30 | |
1st-degree relatives of BRCA mutation carriers but have not been tested | Annually by age 30 but not before age 25 |
Annual MRI beginning at age 30 | |
History of chest irradiation between ages 10–30 | Annually beginning 8 years after radiation therapy but not before age 25 |
Annual MRI beginning 8 years after radiation therapy | |
Lifetime risk ≥ 20–25 %, as defined by models that are dependent on family history (not the Gail model) | Annually by age 30 but not before age 25 or 10 years before the age of the youngest affected relative, whichever is later |
Patients with following genetic syndromes and their first-degree relatives | Annually beginning at age 30–35 years |
Li-Fraumeni | |
Cowden | |
Bannayan-Riley-Ruvalcaba |
Routine preoperative MRI should not be used in all cancer patients since it has no significant impact on outcome. A recent meta-analysis found that preoperative MRI for staging breast cancer did not reduce the risk of local ipsilateral recurrence and distant recurrence [22]. Additionally, preoperative MRI not only fails to reduce re-excisions rate [23], it increases the odds of a patient receiving a mastectomy who would otherwise be a candidate for a BCT [24].
Biopsy Techniques
Breast masses can be cystic, solid, or both. Cystic masses can be aspirated with a fine-needle aspiration (FNA), guided either by palpation or ultrasound (US). Non-bloody aspirate can be discarded unless there is a clinical or radiologic suspicion of an associated malignancy. For non-cystic abnormality, the selection of the most appropriate biopsy technique will depend on whether the abnormality is palpable or non-palpable. Excisional biopsy as the initial diagnostic tool should be avoided unless minimally invasive biopsy techniques (percutaneous image-guided biopsy or palpation-guided) are not available. In general, a percutaneous biopsy such as a core needle biopsy (CNB) with either a 12–14 gauge needle or vacuum-assisted biopsy with a 7–11 gauge needle is the preferred initial diagnostic procedure for both palpable and non-palpable lesions [25]. If the biopsy is positive for either noninvasive cancer (DCIS; see Chap. 3) or invasive cancer, the clinician can then provide the patient with an optimal plan of care, which can include a single trip to the operating room for a definitive surgery. If it is negative or nondiagnostic, then the result should be put in context with the clinical examination and radiologic imaging. If the clinical exam and radiologic imaging are in concordance with the biopsy result (triple concordance), then the likelihood of a missed malignancy is very low (accuracy ranges from 93 to 99 %). However, if there is any discordance (i.e., lesion is suspicious for malignancy based on clinical exam and/or radiologic imaging), then the lesion should be excised for a definitive diagnosis (i.e., excisional biopsy for palpable lesion, needle localization biopsy for non-palpable lesion). Curvilinear incisions following Langer’s skin lines should be considered for optimal cosmesis (Fig. 4.6), and the pathologic specimen should be appropriately oriented. Specimens should be marked in a consistent manner to avoid confusion by the pathologist about the proper orientation, which could influence appropriate re-excision to achieve negative margins. Although a commonly used method calls for a short stitch be placed to designate the superior margin and a long stitch to indicate the lateral margin, this two-stitch method has been shown to be associated with an error rate of 31 % [26]. Intraoperative inking by the surgeon or placement of at least three separate marking sutures/clips for specimen orientation is recommended.
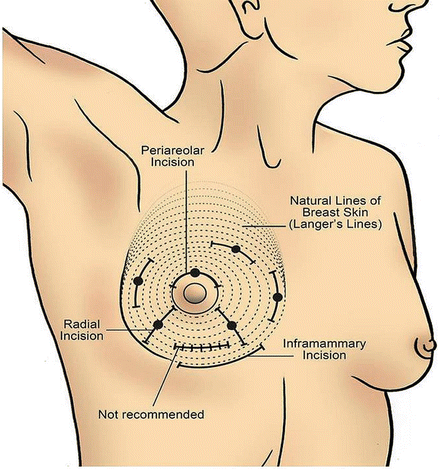

Fig. 4.6
Recommended curvilinear incisions to be used when operating on the breast (Courtesy of Quyen D. Chu, MD, MBA, FACS)
For patients with high-risk lesions such as atypical ductal hyperplasia (ADH), atypical lobular hyperplasia, lobular carcinoma in situ, and radial scars (complex sclerosing lesions) found on percutaneous biopsy, a surgical excision should be performed to assure that no occult DCIS or invasive cancer is present [25]. Of note, if a percutaneous biopsy will remove most or all of the abnormality, then a clip or some other imaging marker should be inserted at the time of biopsy.
Non-palpable Lesions
Besides CNB, stereotactic core biopsy or ultrasound-guided biopsy is also an acceptable modality for non-palpable lesions. For lesion ≤ 1 cm, percutaneous excision using a vacuum-assisted device is recommended [25]. In situations where more tissue is required for a definitive diagnosis, a needle or wire localization biopsy can be performed. Such specimen should be sent to the radiologist to confirm that the abnormal lesion has indeed been excised. Of note, a lesion detected on an ultrasound should be confirmed ex vivo with an ultrasound, while one that was detected on a mammogram should be confirmed ex vivo with a mammogram. If the final specimen is negative for malignancy, our practice has been to repeat the imaging in 6 months.
Palpable Lesions
Although CNB is the preferred initial procedure for palpable lesions, an FNA is also acceptable. In expert hands, the accuracy rate of an FNA ranges from 89 to 98 %. However, the advantage with CNB is that it can differentiate whether the carcinoma is intraductal or invasive, whereas FNA may not because it is limited by few loose cells. For large suspicious lesions (>5 cm), a CNB or an incisional biopsy (taking a small piece of the mass) may be performed to obtain a tissue diagnosis and for determining receptor statuses. This is especially important if neoadjuvant (chemotherapy before surgery) is being considered. Our approach is to send a small piece of the incised mass for frozen section to confirm a diagnosis of cancer before leaving the operating room.
Treatment
Local Control
Once a diagnosis of cancer has been confirmed, the surgeon will need to consider the options of obtaining local control. The primary goal is twofold: (1) resect the primary tumor to negative margins and (2) address the at-risk breast. Options for local control are (1) mastectomy (see Fig. 6.1, Chap. 6) or (2) lumpectomy with negative margins, followed by breast irradiation (XRT), which is also known as breast-conserving therapy (BCT). There is a whole range of radiation options; these include external beam radiation (whole-breast radiation), accelerated partial breast irradiation, 3D conformal radiotherapy, and brachytherapy. External beam radiation is the most common and therefore, unless specified otherwise, most of the discussion on radiation implies the use of this modality.
BCT yields equivalent survival outcome as a mastectomy. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 was the first clinical trial that demonstrated that less radical surgery (i.e., total mastectomy or TM ± radiation) was equivalent to a Halsted radical mastectomy (i.e., removing the breast, the underlying pectoral muscles, and axillary contents) [27–30]. The trial enrolled 1,665 women, and the latest 25-year follow-up results reported in 2002 demonstrated that there were no significant differences in the disease-free survival (DFS), relapse-free survival (RFS), distant-disease-free survival (DDFS), and overall survival (OS) among TM, TM plus radiation, and RM [31] (Table 4.5).
Table 4.5
Selected trials comparing mastectomy and breast-conserving therapy for breast cancer
Trials/authors/ref | N | Mean follow-up (years) | Treatment group | Outcome |
|---|---|---|---|---|
NSABP B-04/Fisher [32] | 1,665 | 25 | (1) TM | No significant differences in DFS, RFS, DDFS, and OS among the three groups |
(2) TM + XRT | ||||
(3) RM | ||||
NSABP B-06/Fisher [33] | 1,843 | 20 | (1) MRM | No significant differences in DFS, DDFS, and OS among the three groups |
(2) L + ALND | ||||
(3) L + ALND + XRT | L alone had higher cumulative incidence of ipsilateral breast cancer recurrence compared to L + XRT (39 % versus 14 %; P < 0.001) | |||
NCI/Jacobson [34] | 247 | 10 | (1) MRM | No significant differences in DFS, OS, or locoregional recurrence rate between the two groups |
(2) L + ALND + XRT | ||||
Milan/Veronesi [35] | 701 | 20 | (1) RM | No significant differences in OS, rates of contralateral breast cancers, distant metastases, or second primary cancers between the two groups |
(2) L + ALND + XRT | L group had significantly higher tumor recurrence rate than the RM group (P < 0.001) | |||
DBCG/Blichert-Toft [36] | 793 | 20 | (1) MRM | No significant differences in local tumor control, RFS, OS between the two groups |
(2) L + ALND + XRT | L had higher rate of new primaries, while MRM had higher rate of true tumor recurrence | |||
EORTC/van Dongen [37] | 903 | NA | (1) MRM | No significant difference in DDS, local-regional recurrence, and OS between the two groups |
(2) L + ALND + XRT | ||||
IGR/Sarrazin [38] | 179 | 10 | (1) MRM | No significant difference in distant metastasis, local-regional recurrence, contralateral breast cancer, and OS between the two groups |
(2) L + ALND + XRT |
Subsequent to the NSABP B-04 trial, the NSABP B-06 trial evaluated 1,843 women with tumors 4 cm or less and randomized them into three groups: TM and ALND (modified radical mastectomy), lumpectomy and ALND with breast irradiation, or lumpectomy and ALND without breast irradiation [33, 39]. The latest 20-year follow-up results, also reported in 2002, found no significant differences in the DFS, DDFS, or OS among the three groups [33]. However, patients who underwent a lumpectomy with breast irradiation had a significantly lower cumulative incidence of ipsilateral breast cancer recurrence (14.3 %) than those who underwent a lumpectomy without breast irradiation (39.2 %) (P < 0.001).
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a meta-analysis of 10,801 women from 17 randomized trials of radiotherapy (XRT) versus no radiotherapy after BCT and found that XRT resulted in a 15.7 % absolute reduction in recurrence at 10 years (2p < 0.00001) and a 3.8 % absolute reduction in breast cancer death at 15 years (2p = 0.00005) compared to no XRT [40]. The magnitude was observed to be higher in node positive than node positive patients (absolute reduction in recurrence, 21.2 % versus 15.4 %, respectively, and absolute reduction in mortality, 8.5 % versus 3.3 %, respectively) [40]. Thus, NSABP B-06 and EBCTCG, along with other clinical trials, established that BCT with radiation is equally effective as a mastectomy and that BCT must be accompanied by XRT [34–36] (Table 4.5).
Role of Accelerated Partial Breast Irradiation
Accelerated partial breast irradiation (APBI) has been explored as an alternative technique to whole-breast irradiation (WBI). The rationale behind APBI is based on data that demonstrated that 80–90 % of local recurrences following a lumpectomy are within the cavity site [31, 41, 42]. Thus, by delivering an intense dose of radiation to the surgical bed, local recurrence rate should be equivalent to WBI. Another advantage with APBI is that it is administered over a shorter period of time (1–2 weeks) instead of the traditional 6-week period with WBI. This is important, especially for women who are candidates for BCT but who happen to reside in a place that is substantially far away from their nearest radiation facility.
APBI comes in different forms, including multicatheter interstitial brachytherapy, intraoperative RT (IORT), external beam conformal therapy (EBRT), and intracavitary balloon brachytherapy such as MammoSite® (Hologic, Inc., Bedford, MA, USA). The American Society of Breast Surgeons (ASBS) MammoSite® Breast Brachytherapy Registry Trial [43] recently published their final analysis on 1,449 patients with early breast cancer who were treated with BCT and MammoSite® and were followed-up for greater than 5 years. Their results, which are similar to others [44, 45], found that the 5-year actuarial ipsilateral breast tumor recurrence (IBTR) rate was 3.8 %, which is in line with historic rate of 2 % for WBI [46]. Additionally, the rate of excellent/good cosmesis at 84 months approached 91 %, and the rates of infectious and noninfectious complications were comparable to WBI historic data [43]. The limitations with this study are the potential for selection bias (surgeons might be reluctant to report complications since it is a volunteer registry), lack of long-term outcomes, and comparison against historic controls.
However, Smith et al. performed a retrospective review of over 92,000 women in the Medicare SEER database and reported that compared to WBI, women who were treated with brachytherapy had a higher mastectomy rate, more frequent infectious and noninfectious complications, and higher incidence of fat necrosis, although the 5-year OS was equivalent between WBI and APBI [47]. The limitations with this study include comparison of WBI with older form of brachytherapy with less control of radiation dose and the use of an administrative database that may be fraught with selection bias, incomplete information, and potential inaccuracy.
Whether APBI is equivalent to WBI is currently the focus of the NSABP B-39/RTOG 0413 clinical trial, a trial that randomizes patients with early stage breast cancer (Stage 0–II) to receive either WBI or APBI. Criteria include patients whose tumor is ≤ 3 cm and who possess no more than three histologically positive lymph nodes (Table 4.6). Unfortunately, results will not be known for another 5–10 years [48].
Table 4.6
NSABP B-39/RTOG 0413 criteria for clinical trial comparing accelerated partial breast irradiation (APBI) with whole-breast radiation (WBI) in women with early breast cancer
Patients with Stage 0, I, and II breast cancer resected by lumpectomy |
Tumor size ≤ 3 cm |
No more than 3 histologically positive nodes |
The decision to perform BCT depends on many factors, one of which is cosmesis. If the lumpectomy will result in a disfigurement of the remaining breast, it is better to opt for a mastectomy. In addition, BCT is ideal for patients who are compliant, are not pregnant, have access to a radiation facility, have no history of radiation therapy to the chest wall (i.e., history of radiation for lymphoma), and have no history of collagen vascular disease (i.e., scleroderma and systemic lupus erythematosus).
One of the tenets in breast surgery is the need to achieve a negative margin to avoid local recurrence (LR). What constitutes an adequate negative margin, however, remains an area of great interest. According to NSABP, a negative margin is defined as having no tumor at the inked surgical margin, irrespective of the distance from the nearest tumor cell. In a meta-analysis, of 21 studies and over 14,000 women with early breast cancer who underwent BCT, Houssami et al. confirmed that those with positive margins (presence of any cancer, invasive and/or DCIS at the inked margin) had a significantly higher likelihood of having LR (odds ratio of 2.42; P < 0.001) compared to those with negative margins [49]. The margin width (1 mm versus 2 mm versus 5 mm), however, had no significant impact on the rate of LR. The authors conclude that it is reasonable to define a minimum distance of 1 mm for negative margins in women who undergo BCT for early breast cancer [49]. Finally, a panel of breast experts recently developed a guideline for defining adequate margins in the setting of BCT and adjuvant radiation therapy and concluded that no ink on tumor should suffice as adequate margin in invasive cancer [50].
To summarize this portion of the discussion, both a mastectomy and a BCT will achieve equivalent local control. In a mastectomy, the tumor and the at-risk breast are removed at the same setting, whereas in BCT, the tumor is resected and the at-risk breast is irradiated. A negative margin is a negative margin. The role of APBI will be clarified, based on the results of the NSABP B-39/RTOG 0413 clinical trial.
Regional Control
Once a local control option has been selected, the next goal is to obtain regional control. Although there is a rich network of lymphatics that drains the breast, surgeons generally focus their attention on the axillary lymph nodes for evidence of metastases. Lymph node status is one of the most important prognostic factors in breast cancer [51]. Options for assessing the lymph nodes are sentinel lymph node biopsy (SLNBx) or axillary lymph node dissection (ALND); the latter, when combined with a mastectomy, is a modified radical mastectomy (MRM) (Fig. 4.7a–d).
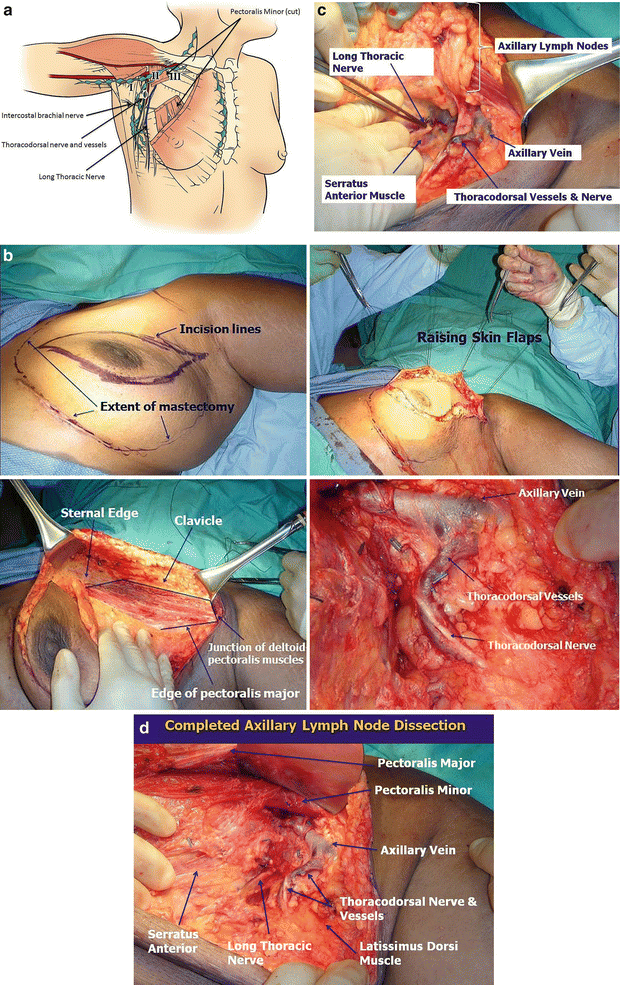

Fig. 4.7
A modified radical mastectomy (MRM) requires a thorough knowledge of breast anatomy (a). In general, the following nerves should be preserved: the long thoracic nerve, which innervates the serratus anterior and lies along the muscle. Injury to this nerve can result in a “winged scapula.” The thoracodorsal nerve generally runs along the thoracodorsal vessels and at a right angle to the axillary vein. The nerve innervates the latissimus dorsi, and an injury to it results in the patient being unable to raise the trunk with the upper limb. Finally, the intercostal brachial nerve supplies sensation to the back of the arm. Its injury can lead to a feeling of “numbness” in the upper medial aspect of the arm. The levels of the lymph nodes are named based on their relationship to the pectoralis minor muscle. Axillary lymph node dissection generally involves removal of level I lymph nodes (lateral to the pectoralis minor muscle) and level II lymph nodes (underneath/posterior to the pectoralis minor muscle). Level III lymph nodes (above and medial to the pectoralis minor muscle) are removed when they are palpable. This generally requires resecting the pectoralis minor muscle at its insertion into the coracoid process. (b) A modified radical mastectomy begins with an elliptical incision that incorporates the nipple/areolar complex. The superior and inferior flaps are raised and the breast tissue is removed. Note that the axillary contents are below the axillary vein and that the thoracodorsal nerve tends to run parallel to the thoracodorsal vessels. (c) This figure shows the major structures just before the axillary contents are removed. (d) Picture of a completed axillary lymph node dissection. Note the long thoracic nerve along the serratus anterior muscle (a–d: Courtesy of Quyen D. Chu, MD, MBA, FACS)
Historically, ALND was a routine component of the management of the majority of patients with early breast cancer. The procedure, however, does carry some inherent risks such as neurovascular injury (injury to the axillary vessels or long thoracic nerve), seroma formation, decrease in range of motion in the shoulder, and chronic lymphedema. Minimizing these risks without compromising oncologic principles became a major focus of the medical community. Sentinel lymph node biopsy became an alternative technique to assess the axilla for a subset of patients. The principle behind SLNBx is that when breast cancer metastasizes to the lymph nodes, it does so in an orderly manner. Because breast cancer generally drains to the first echelon of lymph nodes before draining to the subsequent echelons of lymph nodes, the likelihood of having metastases in subsequent echelons will be low if there is no evidence of metastases in the first echelon of lymph nodes.
SLNBx removes only a small number of lymph nodes and therefore requires less dissection in the axilla. Several studies have demonstrated that SLNBx is less morbid [52, 53] and yielded equivalent outcome as an ALND [54–58] (Table 4.7). Sentinel lymph node identification rate ranges 95–98.7 %, and the false-negative rate is generally less than 10 % [55–57]. Most surgeons use both isosulfan blue (blue dye) and technetium-99 sulfur colloid (double-tracer technique) to identify the sentinel nodes (Fig. 4.8a, b). Injection can be done either peritumorally or subareolar [59], the latter being done in situation when the tumor is not palpable (Fig. 4.9). To achieve an acceptable false-negative rate (FNR) threshold of 10 %, at least two sentinel nodes should be retrieved. NSABP B-32 reported an FNR of 18 % with 1 SLN resected, 10 % with 2 SLNs resected, and 7 % with 3 SLNs resected [60]. When the sentinel node(s) cannot be identified, a standard ALND should be performed. On occasions, internal mammary lymph node(s) (IMN) may be seen on lymphoscintigraphy, especially for cancers located in the medial aspect of the breast. Most surgeons do not perform IMN biopsy or dissection due to the associated morbidity; instead, the IMN is generally treated with radiation.
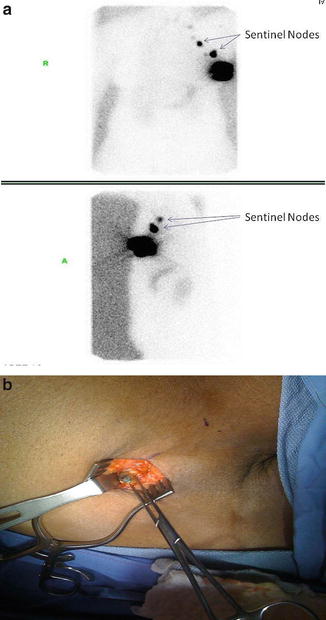
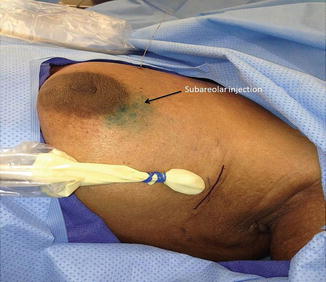
Table 4.7
Selected series comparing sentinel lymph node biopsy with axillary lymph node dissection
Author/year | # Patients | Median follow-up (mos) | Axillary recurrence rate | 5-year DFS | 5-year OS | |||
|---|---|---|---|---|---|---|---|---|
SLNBx | ALND | SLNBx | ALND | SLNBx | ALND | |||
516 | 102 | 0 % | 2 % | 89.9 % | 88.9 % | 93.5 % | 89.7 % | |
p = 0.17 | p = 0.52 | p = 0.15 | ||||||
Zavagno [56] | 749 | 56 | 0.29 % | 0 % | 87.6 % | 89.9 % | 94.8 % | 95.5 % |
(Sentinella/GIVOM) 2008 | p = NA | p = 0.77 | p = NA | |||||
Krag (NSABP-B32) [57] | 5,611 | 95.6 | 0.7 % | 0.4 % | 88.6 % | 89.0 % | 95.0 % | 96.4 % |
2010 | (mean) | p = 0.22 | p = 0.54 | p = 0.12 | ||||
Giuliano [58] | 891 | 76 | 0.9 % | 0.5 % | 83.9 % | 82.2 % | 92.5 % | 91.8 % |
(ACOSOG Z0011) 2011 | p = 0.14 | p = 0.25 | ||||||

Fig. 4.8
Sentinel lymph node mapping of a breast cancer using lymphoscintigraphy (a). Radiotracer used was Tc-99 m sulfur colloid. Sentinel node was identified with isosulfan blue (blue dye) (b) (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 4.9
Subareolar injection can be performed to identify sentinel nodes. This can be done in situations when the tumor is not palpable (Courtesy of Quyen D. Chu, MD, MBA, FACS)
ACOSOG Z0010 and NSABP B-32 reported that SLN disease detected on IHC has no clinical significance and, therefore, recommended that IHC staining of SLNs is unnecessary [61, 62]. Thus, hematoxylin and eosin staining of SLN is all that is needed, and given the recent ACOSOG Z0011 data, intraoperative frozen-section analysis of the SLN should be avoided for nodes that are not highly suspicious, especially if the patient meets ACOSOG Z0011 criteria.
Should a Completion ALND Be Performed for Low-Volume Axillary Disease?
Sentinel lymph node biopsy is now the standard of care for most women with invasive breast cancer and indicated for virtually any patient with clinically node-negative invasive breast cancer [63]. The American Society of Clinical Oncology (ASCO) recommends SLNBx in patients with T1 and T2 tumors and clinically node-negative disease [64]. It also supports SLNBx for those with multicentric tumors and with ductal carcinoma in situ (DCIS) who opted for a mastectomy since an SLNBx will be impossible to perform if an invasive tumor is found in the mastectomy specimen [64]. For patients who have palpable axillary lymphadenopathy or are pregnant, or for an SLNBx that cannot be identified, or if the technique is not available, an axillary lymph node dissection (ALND) should be performed (Table 4.8).
Table 4.8
Indications and contraindications for sentinel lymph node biopsy
Indications: clinically negative axilla and |
1. T1/T2 tumors |
2 DCIS in women who opted for a mastectomy |
Controversial indications |
1. Multicentric tumor |
2. Prior axillary surgery |
3. T3/T4 |
Absolute contraindications |
1. Clinically palpable axillary lymphadenopathy |
2. Large T4 tumors |
3. Inflammatory breast cancer |
4. Pregnant women |
For patients with a negative SLNBx, no further surgery in the axilla is needed. However, how should a patient with positive SLNBx be managed? Traditionally, such a patient undergoes a completion ALND because the incidence of additional disease on ALND can be as high as 53 % [65]. In the past, several predictive nomograms were developed to estimate the risk of additional positive nodes in the hope of sparing women the morbidity of unnecessary ALND [66, 67]. However, such nomograms will likely be supplanted by recent data from the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial.
ACOSOG Z0011 demonstrated that a completion ALND might not be necessary in a subset of patients who have positive SLNBx [58]. ACOSOG Z0011 was a phase III non-inferiority trial that randomized 891 women who had positive sentinel lymph nodes to either observation (N = 446) or undergo a completion ALND (N = 445). All women had BCT, clinical T1–T2 tumor, systemic therapy as appropriate, and whole-breast irradiation (WBI) with tangential fields (TFs) which partially covers level 1 and 2 lymph nodes. At a median follow-up of 6.3 years, regional recurrence was less than 1 % with SLNBx alone, despite 27 % of patients having had additional metastases in the undissected axillary nodes (based on indirect evidence that 27 % of patients in the ALND group had nodal disease). The 5-year OS for SLNBx alone versus ALND group was 92.5 % and 91.8 %, respectively (p = 0.25), and the 5-year DFS for SLNBx alone versus ALND group was 83.9 % and 82.2 %, respectively (p = 0.14). On multivariate analysis, neither the number of positive SLNBx, size of SN metastasis, nor number of LNs removed was associated with locoregional recurrence. This may be due to the effectiveness of systemic therapy and TF irradiation to eliminate low-volume residual nodal disease.
Thus, based on Z0011, SLNBx alone is adequate for patients who meet all of the following three criteria: (1) small tumor (T ≤ 5 cm), (2) two sentinel lymph nodes or fewer are positive, and (3) the patient will undergo a lumpectomy with adjuvant systemic therapy and radiation to the axilla (Table 4.9). It must be cautioned that patients who have a mastectomy or those who will not receive radiation that covers the axilla (i.e., partial breast irradiation) will still require an ALND for a positive sentinel lymph node biopsy.
Table 4.9
ACOSOG Z0011 criteria precluding the need for a completion axillary lymph node dissection in patients with positive sentinel lymph node biopsy
1. T ≤5 cm |
2. ≤2 positive sentinel lymph nodes |
3. Lumpectomy with whole-breast irradiation |
4. Receiving systemic therapy |
The International Breast Cancer Study Group Trial 23-01 (IBCSG 23-01) also reported similar results as the Z0011 trial. IBCSG randomized 931 patients with non-palpable axilla whose tumor was ≤ 5 cm and SLNBx with one or more micrometastasis (≤2 mm) to either observation or completion ALNDs. Similar to Z0011, less than 1 % recurred in the axilla in the observation group, and there was no significant difference in either DFS or OS between the groups [68].
What Is the Role of Preoperative Ultrasound-Guided Biopsy of the Axilla?
Preoperative axillary ultrasound combined with US-guided axillary lymph node biopsy has been championed by some clinicians because it avoids having the patient undergo a two-step axillary surgery (SLNBx followed by completion ALND). However, given the recent ACOSOG Z0011 data, such a practice may be limited to a select group of patients. US evaluation may have a role in those who will undergo a mastectomy, since patients with an US-guided biopsy-proven axilla can undergo a one-step surgery (completion ALND). However, patients with a negative US-guided biopsy should undergo an SLNBx since a recent meta-analysis found that one in four women with a US-guided biopsy-proven negative axilla has a positive SLNBx [69].
Role of SLNBx in Patients Who Undergo Neoadjuvant Chemotherapy
Although SLNBx is an acceptable option for patients with clinically node-negative breast cancer who will undergo primary surgery, its role and timing for patients who undergo neoadjuvant chemotherapy is unclear. For those who present with clinically negative lymph node (cN0), the options are either SLNBx before or after neoadjuvant chemotherapy. Pooled analysis of data in the literature suggests that SLNBx after neoadjuvant chemotherapy for cN0 is an acceptable diagnostic procedure because the sentinel lymph node identification rate and false negative rate (FNR) are comparable to patients who undergo SLNBx before chemotherapy [70]. However, for patients with cN0 but a positive SLNBx who opted for neoadjuvant therapy, an ALND should be performed since the FNR for a second SLNBx following neoadjuvant chemotherapy is above 50 % [71].
What is the reliability of performing an SLNBx following neoadjuvant therapy for those who present with clinically positive nodal disease (cN+)? ACOSOG Z1071 (Alliance) [72] and SENTinel NeoAdjuvant (SENTINA) [71] evaluated patients with cN + who underwent SLNBx following neoadjuvant chemotherapy and found that the procedure resulted in a high FNR. For those that had two or more SLNBs retrieved, the FNR was 12.6 % in the ACOSOG Z1071 trial. Even if the patient’s axilla was downstaged to cN(−) following neoadjuvant chemotherapy, the FNR was 18.5 %, as reported in the SENTINA trial. Thus, the results of both trials exceeded the 10 % threshold. Because of this, the authors concluded that SLNBx following neoadjuvant chemotherapy for patients with cN(+) is not a reliable alternative over ALND (Table 4.10).
Table 4.10
Trials evaluating role of sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer
Trials/author/year/ref | N | False-negative results (based on # LNs retrieved) |
|---|---|---|
ACOSOG Z1071 | 663 | 1 SLN: 31.5 %a |
Boughey/2013 [72] | 2 SLNs: 21 % | |
≥ 3 SLNs: 9 % | ||
SENTINA/Kuehn/2013 [71] | 1,737 | 1 SLN: 24.3 % |
2 SLNs: 18.5 % | ||
≥ 3 SLNs: <10 % |
Can Regional Nodal Irradiation Substitute ALND?
The European Organization for Research and Treatment of Cancer (EORTC) After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) was a phase III trial comparing ALND with axillary radiation therapy (ART) in women with EBC tumor [73, 74]. Nearly 5,000 women with tumor < 3 cm and positive SLNBx were randomly assigned to either a completion ALND or ART, the latter encompasses all three levels of the axilla and the medial part of the supraclavicular fossa. Systemic therapy was given as appropriate and 80 % of patients had BCT. With a follow-up beyond 10 years, the final analysis, which was presented at the 2013 Annual Meeting of the American Society of Clinical Oncology (ASCO), found that compared to ALND, ART resulted in equivalent 5-year OS (93.27 % ALND, 92.52 % ART, p = 0.338) and 5-year DFS (86.9 % ALND, 82.65 ART, p = 0.178). The rate of regional recurrence was 0.43 % in the ALND arm and 1.19 % in the ART arm. However, lymphedema was twice as high in the ALND as in the ART arm [74]. Notwithstanding, this data makes the management of patients with positive SLNBx more complex. Should patients with a small tumor and one positive SLNBx who opted for BCT be offered conventional radiation with tangential field or nodal regional irradiation, the latter would be considered by many as overtreatment? Complications from regional nodal irradiation include pain, pneumonitis, lymphedema, brachial plexus neuropathy, malignancy, radiation dermatitis, and poor cosmetic outcome.
As an aside, both the Z0011 and AMAROS use the non-inferiority test rather than a superiority test. Such a test requires fewer patients and can often fail to detect small significant differences. Thus, longer follow-up will be necessary to understand the impact of the experimental arm.
Should Regional Nodal Irradiation Be Given to High-Risk Early Breast Cancer?
There is a group of patients with EBC who are at high risk of developing recurrent disease, despite optimal WBI. The NCIC Clinical Trials Group MA.20 (NCIC CTG MA.20) trial evaluated the role of adding regional nodal irradiation (RNI) to WBI in high-risk women who underwent BCT for EBC and received adjuvant systemic therapy. High-risk groups include node-positive patients and node-negative patients who have tumor ≥ 5 cm or if it was ≥ 2 cm and fewer than 10 lymph nodes removed, with either ER-negative, nuclear grade 3, or evidence of lymphovascular invasion [75]. Over 1,800 women were randomized to conventional WBI or WBI + RNI; RNI included the internal mammary, supraclavicular, and high axillary lymph nodes. The interim analysis found that after a median follow-up of 62 months, WBI + RNI significantly reduced locoregional recurrence from 5.5 to 3.2 % (HR = 0.58; P = 0.02) and distant recurrence from 13.0 to 7.6 % (HR = 0.64; P = 0.002). The DFS was better in the WBI + RNI group (89.7 %) than the WBI group (84 %; P = 0.003), although OS was not significantly different (92.3 % WBI + RNI versus 90.7 % WBI; P = 0.07) [75].
Given the two options for local control and two options for regional control, on any given patient, there can be up to four options for achieving locoregional control: (1) lumpectomy/XRT + SLNBx, (2) lumpectomy/XRT + ALND, (3) mastectomy + SLNBx, and (4) mastectomy + ALND, also known as a modified radical mastectomy (MRM).
Should Postmastectomy Radiation Be Given to High-Risk Early Breast Cancer?
Postmastectomy radiation (PMRT) is known to be of benefit in patients with locally advanced breast cancer and those with ≥ 4 positive LNs [76, 77]. However, its role in those with T1–2 and limited nodal disease (1–3 positive LNs) remains controversial. Retrospective data showed that among patients with T1–2 tumors and 1–3 positive axillary lymph nodes, postmastectomy radiation reduces LRR rate especially in those who are ≤ 50 years or have evidence of lymphovascular invasion (LVI) [78, 79]. PMRT can also be considered in those with 1–3 involved nodes with large tumors, extranodal extension, or inadequate axillary dissections.
Distant Control
The majority of recurrences occur during the first 2 years following treatment. The risk of recurrences averages around 4 % per year after 5 years [80]. Distant control is of utmost importance and will require the assistance of colleagues who have expertise in the field of medical oncology. Note that in managing patients with early breast cancer, the surgeons directly impact local and regional control. She/he cannot excise potential blood stream metastases with her/his scalpel. Distant control will require systemic therapy (adjuvant therapy), either in the form of chemotherapy, hormonal therapy, and/or biologic therapy. A patient with early breast cancer may be a candidate for any combination of these therapies.
Adjuvant therapy is therapy following definitive surgery, while neoadjuvant or preoperative therapy is therapy before definitive surgery. The course of therapy chosen is predicated mainly on the characteristics of the primary tumor, although other factors such as the patient’s age, comorbidities, and risk of relapse also are considered in the final plan of therapy. Besides confirming the tumor histology, the pathologist will provide many key information; among them are tumor size (T status), margin status, grade, nodal status (N status) (i.e., the number of lymph nodes involved in relation to the total number of lymph nodes retrieved), and receptor statuses for estrogen receptor (ER status), progesterone receptor (PR status), and HER-2.
When evaluating the efficacy of a therapy, it is important to have a clear understanding of several important statistical concepts, mainly hazard ratio (HR), absolute risk reduction (ARR), and relative risk reduction (RRR); the last is often referred to as a proportional risk reduction. All three metrics can be converted and reported as percentages. The ARR is the absolute difference between the curves at a selected time period, while the RRR is calculated as one minus the relative risk (RR) times 100 (Fig. 4.10). For instance, in the example given in Fig. 4.10, the ARR is 5 % and the RRR is 16 %. If one were to ask the uninformed individual the question of whether he/she prefers to have an ARR of 5 % or a RRR of 16 %, most would choose the latter. However, the correct answer would be that it does not matter because the two are equivalent.
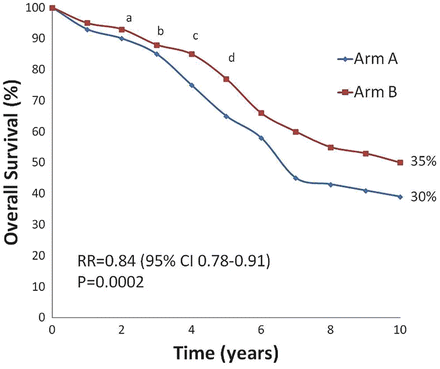

Fig. 4.10
Absolute risk reduction, relative risk reduction (RRR), and hazard ratio (HR): note that arm B resulted in a significantly improved overall survival compared to arm A. Compared to arm A, treatment with arm B resulted in the absolute risk reduction of 5 % at 10 years (35–30 %) and the relative risk reduction of 16 % (RRR = 1-RR*100 or 1-0.84 * 100). Another important concept is the hazard ratio (HR), which is the time-to-event analysis or an instantaneous event rate. HR equals to the hazard in the treatment arm divided by the hazard in the control arm. HR measures the probability that an individual has an event (i.e., recurrence) at a particular time period. For instance, if the HR is 0.34, then the participants in the treatment arm B have a 66 % (1–0.34 × 100) reduction in having an outcome (i.e., death) compared to the control arm A at any particular time along the follow-up period (points a–d) (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Another important statistical concept is the hazard ratio (HR), which is the time-to-event analysis or an instantaneous event rate. HR equals to the hazard in the treatment arm divided by the hazard in the control arm. HR measures the probability that an individual has an event (i.e., recurrence) at a particular time period. For instance, if the HR is 0.34, then the participants in the treatment arm have a 66 % (1–0.34 × 100) reduction in having an outcome (i.e., recurrent disease) compared to the control arm at any particular time along the follow-up period. Although similar to relative risk, they are not the same. RR does not care about events along the time period, but rather measures the total number of events at the end of the study.
Adjuvant Chemotherapy
Adjuvant chemotherapy reduces breast cancer recurrence and mortality by approximately 30 % and 20 %, respectively [81]. The 20 % absolute improvement in survival rate was the result of small incremental improvements made over the decades, beginning with a 4 % improvement with CMF, 4 % with anthracyclines, 5 % with taxanes, and finally up to 8.8 % with trastuzumab.
Randomized trials performed prior to the era of systemic chemotherapy demonstrated a 10-year overall survival of 60 % for patients with operable breast cancer [82]. Clearly, there was room for improvement. The NSABP B-01 trial was the first to demonstrate the effectiveness of using single agent chemotherapy (thiotepa) in the adjuvant setting [83, 84]. NSABP B-05 was a confirmatory trial but used melphalan instead of thiotepa [85]. Combination regimen with cyclophosphamide, methotrexate, and fluorouracil (CMF) was first introduced by the Italian group at the Istituto Nazionale Tumori (ITA) of Milan [86] and the efficacy of polychemotherapy in patients with EBC was confirmed by many studies, including the meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) [87, 88]. Anthracycline-based chemotherapy (i.e., adriamycin or epirubicin) entered the scene in the early 1980s, and various anthracycline-based combinations (AC, FEC, FAC, etc.) were subsequently demonstrated to offer a significant, albeit small, advantage over non-anthracycline-based chemotherapy (CMF) [88, 89]. Compared to CMF, anthracycline-based therapy resulted in a significant reduction in recurrence and breast cancer mortality; the 10 year relative risk reduction in recurrence and mortality was 11 % and 20 %, respectively, and the absolute reduction in recurrence and breast cancer mortality was 2.6 % and 4.1 %, respectively [88].
In the early 2000s, taxanes were tested against the classic anthracycline (AC or EC) regimen in high-risk patients, mainly those with node-positive EBC. Taxanes are potent agents that bind to and stabilize microtubules, thereby preventing their depolymerization. Paclitaxel (Taxol®) and docetaxel (Taxotere®) are the two most actively tested agents. Multiple clinical trials demonstrated the superiority of adding taxanes to the regimen in high-risk early breast cancer patients [90]. A recent meta-analysis of 14 randomized phase III studies of over 25,000 patients demonstrated improved DFS and OS with docetaxel-containing regimen over non-docetaxel-containing regimen in node-positive patients [90]. Similarly, the recent 2012 EBCTCG meta-analysis of outcomes among 100,000 women in 123 randomized trials of adjuvant chemotherapy reported that compared to non-taxane anthracycline regimen, the taxane + anthracycline regimen resulted in a significant 8-year relative risk reduction of recurrence and breast cancer mortality by 16 % and 14 %, respectively. This translates to an absolute benefit of 4.6 % and 2.8 % for breast cancer recurrence and breast cancer mortality, respectively [88].
Is Taxane Indicated in High-Risk Node-Negative Patients?
Although taxane-based adjuvant therapy is standard of care for patients with node-positive breast cancer, its use in node-negative breast cancer remains controversial [91, 92]. The Spanish Breast Cancer Research Group (GEICAM) 9805 trial randomly assigned 1,060 women with node-negative operable breast cancer who have at least one high-risk factor for recurrence according to the 1998 St. Gallen criteria (tumor size ≥ 2 cm, TNBC, grade 2 or 3, or age < 35 years) to either standard chemotherapy (fluorouracil, doxorubicin, and cyclophosphamide-FAC) or taxane-based therapy (docetaxel, doxorubicin, and cyclophosphamide-TAC) [91]. At a median follow-up of 77 months, there was a 32 % reduction in the risk of recurrence when docetaxel was added to the standard AC regimen (P = 0.01). However, there was no significant difference in OS between the two groups (P = 0.29), and more significant grade 3 or 4 toxicities were observed in the docetaxel group (P < 0.001) [91]. Similar results were also observed in a trial conducted by the European Cooperative Trial in Operable Breast Cancer [92] as well as a recent meta-analysis [90].
Should All Patients Be Offered Adjuvant Chemotherapy?
Despite its effectiveness, chemotherapy does have its inherent toxicities; approximately 1 % of patients have life-threatening toxicities [93]. Historically, the decision to offer adjuvant chemotherapy relies on clinicopathologic factors such as age, tumor size, axillary lymph node status, pathologic grade, hormone receptor status, and HER-2 status. While these factors are useful in treatment decisions, they are imperfect. Nodal status, traditionally regarded as the most powerful prognosticator in breast cancer, either overestimates or underestimates outcome in 20–30 % of patients; up to 20 % of node-negative patients may develop systemic disease whereas 30 % of node-positive patients may not [94, 95]. Molecular profiling of tumors to search for a “molecular signature” of aggressiveness may provide useful prognostic and predictive information [96, 97]. Of note, prognostic factors predict patient outcome regardless of the treatment given (i.e., tumor size, nodal status), whereas predictive factors indicate responsiveness to a specific treatment (i.e., ER/PR status) [98]. Some factors such as ER/PR and HER-2 are both prognostic and predictive. Predictive factors are complimentary to prognostic factors in that it can be used to select appropriate additional therapy for a patient when necessary.
Many prognostic studies grouped patients as either belonging to the low-risk group or high-risk group, although some also include an intermediate-risk group. In general, high risk is defined as a risk of recurrence of greater than 10 % at 5 years and include those that have nodal involvement [N (+)], HER-2-positive disease [HER-2 (+)], ER-negative disease [ER (−)], triple-negative breast cancer [TNBC or ER(−), PR(−), HER-2(−)], high grade, and high proliferation index as measured by Ki-67. Some would also consider node-negative disease with tumor measuring greater than 1 cm as belonging to the high-risk group [99], although such a low threshold might lead to overtreatment.
There are a number of prognostic indexes that separate the low-risk from the high-risk group. Three clinicopathologic indices will be highlighted, while the three major molecular profiling platforms (Oncotype DX®, MammaPrint®, Mammostrat®) will be discussed under the Prognostic Indices to Identify Low-Risk Estrogen Receptor-Positive, Node-Negative Patients section. All indices have their strengths and weaknesses.
The three prognostic indices that use clinicopathologic features are the Nottingham Prognostic Index (NPI) [100], Adjuvant!Online (AOL) [101], and the St. Gallen guidelines [102]. The Adjuvant!Online (AOL) is a web-based actuarial tool that incorporates four tumor characteristics (tumor size, tumor grade, nodal status, ER status) and one patient characteristic (age) in order to predict patient outcome at 10 years. The St. Gallen uses six tumor characteristics (tumor size, tumor grade, nodal status, ER/PR status, HER-2 status, degree of peritumoral vascular invasion) and one patient characteristic (age) and categorizes patients into low risk, intermediate risk, and high risk; it is commonly used in Europe. The Nottingham Prognostic Index (NPI) takes into account three tumor characteristics (tumor size, tumor grade, and nodal status), and like the AOL, it predicts the 10-year survival rate based on a score; the lower the score, the higher the survival rate. Note that all three indices incorporate tumor size, nodal status, and tumor grade (Table 4.11).
Table 4.11
Prognostic platforms
Authors/ref | Type of indices | Platform |
|---|---|---|
Adjuvant!Online (AOL) | Clinical | Tumor size |
Nodal status | ||
Tumor grade | ||
ER status | ||
Age | ||
Nottingham Prognostic Index (NPI) | Clinical | Tumor size |
Nodal status | ||
Tumor grade | ||
St. Gallen | Clinical | Tumor size |
Nodal status | ||
Tumor grade | ||
ER/PR status | ||
Age | ||
HER-2 Status | ||
Degree of peritumoral vascular invasion | ||
Paik | Oncotype DX (RS) | Low risk: RS <18 |
Intermediate risk: RS 18–30 | ||
High risk: ≥31 | ||
Jankowitz | BCI | HOXB13:IL17BR (H:I) and 5-gene molecular grade index (MGI) |
MammaPrint® | 70-gene signature | Low risk |
High risk | ||
Mammostrat® | SLC7A5, p53, HTF9C, NDRG1, CEACAM5 | Low risk: (≤0) |
Moderate risk: (>0 and ≤0.7) | ||
High risk: >0.7 | ||
RxPONDER Trial | Oncotype DX (RS) | Node (+) (1–3 positive nodes) |
Hormone (+)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|