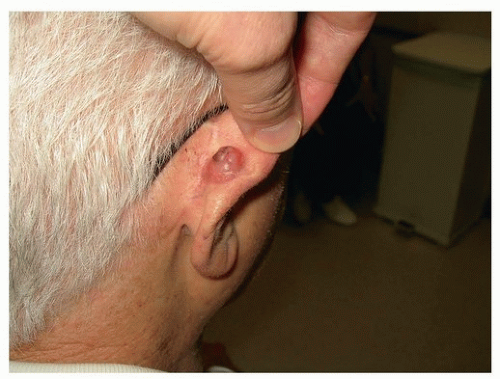
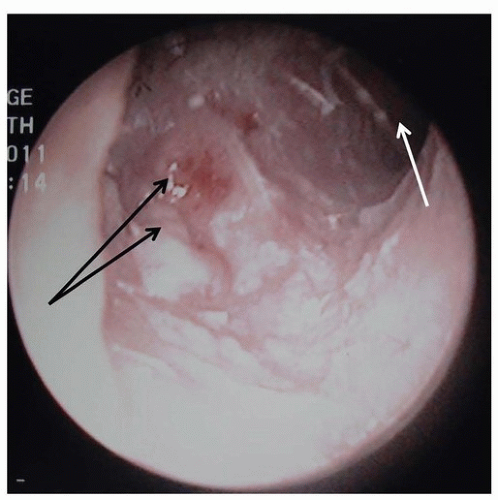
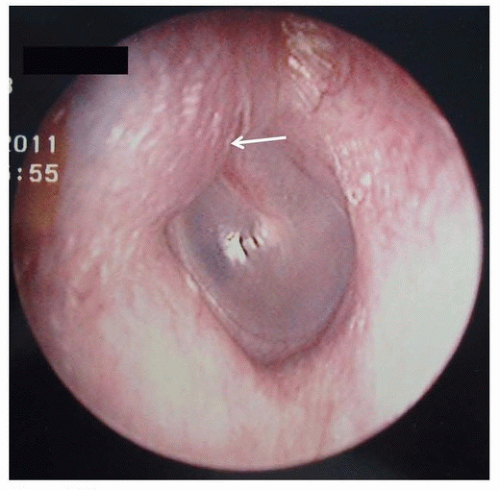
made up 55% to 67% of malignancies affecting the outer ear, whereas BCC made up 28% to 32% and melanoma was 1% to 5%.17
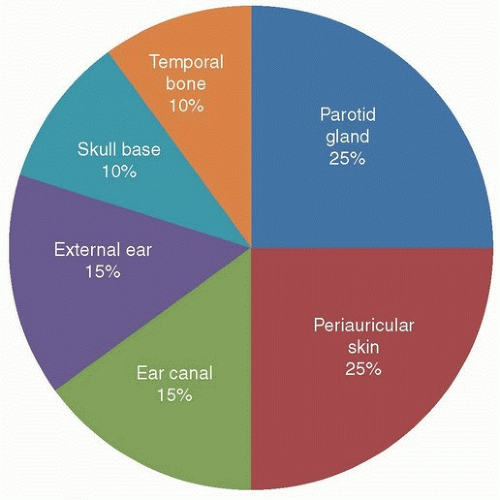 Figure 23.1. Pie chart representation of location of temporal bone primary tumors. (Used with permission, Department of Head and Neck Surgery, MD Anderson Cancer Center.) |
Table 23.1 Histologic Types of External Ear Cancers at University of Texas MD Anderson Cancer Center Between 1990 and 2006 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
of invasion of tumors and perhaps contribute to the insidious and profound invasion that is a notorious hallmark for this anatomic site.5 Equally, the embryologic compartments from each hillock might explain why tumor spread is limited in the early stages of the disease.59
Table 23.2 List of Malignant Tumor Types Found Affecting the Temporal Bone | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Table 23.3 Sites of External Ear Involvement from Squamous Cell Carcinoma (SCC), Basal Cell Carcinoma (BCC), and Melanoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
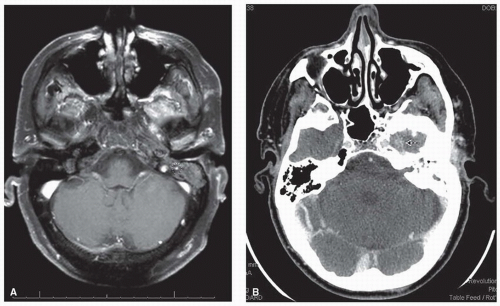
adjunct especially in cases where dural involvement or perineural invasion is suspected (Fig. 23.8).
T2 regardless of size. An external ear cancer that invades the temporal bone is considered T3. Perineural invasion of the skull base (e.g., facial or trigeminal nerve) is considered T4.
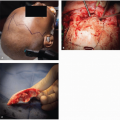
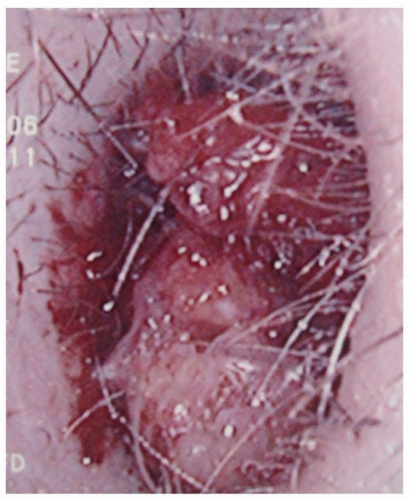 Figure 23.5. SCC filling the left ear canal. (Used with permission, Department of Head and Neck Surgery, University of Texas MD Anderson Cancer Center.) |
Location on or around the external ear as an independent risk factor for metastatic spread.9,15,85,86 The metastasis rate for the external ear was 11.0% versus 5.2% for sunexposed skin.86
Horizontal size.85,86,87,88 TNM staging uses 2 cm to separate T1 from T2 lesions. The NCCN guidelines consider any ear
tumor ≥6 mm in size to be a high-risk lesion for recurrence and metastasis.50
Table 23.4 TNM Staging for Cutaneous Carcinomas
Primary Tumor (T)
TX
Primary tumor cannot be assessed.
T0
No evidence of primary tumor
Tis
Carcinoma in situ
T1
Tumor ≤2 cm in greatest dimension with <2 high-risk featuresa
T2
Tumor >2 cm in greatest dimension
OR
Tumor any size with ≥2 high-risk featuresa
T3
Tumor with invasion of maxilla, mandible, orbit, or temporal bone
T4
Tumor with invasion of skeleton or perineural invasion of skull base
Regional Lymph Nodes (N)
NX
Regional lymph nodes cannot be assessed.
N0
No regional lymph node metastases
N1
Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension
N2
Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension; or in multiple ipsilateral lymph nodes, ≤6 cm in greatest dimension; or in bilateral or contralateral lymph nodes, ≤6 cm in greatest dimension
N2a
Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension
N2b
Metastases in multiple ipsilateral lymph nodes, ≤6 cm in greatest dimension
N2c
Metastases in bilateral or contralateral lymph nodes, ≤6 cm in greatest dimension
N3
Metastasis in a lymph node, >6 cm in greatest dimension
Distant Metastasis (M)
M0
No distant metastases
M1
Distant metastases
a High-risk features for T stage: (1) depth/invasion >2 mm thickness (Breslow thickness), Clark level ≥ IV, or positive perineural invasion, (2) poorly differentiated or undifferentiated, or (3) anatomic location of the ear.
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC, www.springer.com.
Table 23.5 Modified Pittsburgh Staging System19 for Squamous Cell Carcinoma of the Temporal Bone
T Classification
Description
T1
Limited to the EAC without bony erosion or evidence of soft tissue involvement
T2
Limited to the EAC with bone erosion (not full thickness) or limited soft tissue involvement (<0.5 cm)
T3
Erosion through the osseous EAC (full thickness) with limited soft tissue involvement (<0.5 cm) or tumor involvement in the middle ear and/or mastoid
T4
Erosion of the cochlea, petrous apex, medial wall of the middle ear, carotid canal, jugular foramen, or dura; with extensive soft tissue involvement (>0.5 cm, such as involvement of the TMJ or styloid process); or evidence of facial paresis
N Classification
N0
No regional nodes involved
N1
Single metastatic regional node <3 cm in size
N2
N2a
Single ipsilateral metastatic node 3-6 cm in size
N2b
Multiple ipsilateral metastatic lymph nodes
N2c
Contralateral metastatic lymph node
N3
Metastatic lymph node >6 cm in size
Overall Stage
I
T1N0
II
T2N0
III
T3N0
IV
T4N0 and T1-4N+
EAC, external auditory canal; TMJ, temporomandibular joint; N+, any positive lymph node.
Tumor thickness, more than 4 mm.40,85,86,89 In a prospective study of 615 patients with cutaneous SCC, nodal metastasis occurred in 4% of patients with tumors 2.1 to 6.0 mm in thickness and in 6% of patients with tumors thicker than 6.0 mm.85 Another study of 509 patients found that a tumor thickness of 5 mm is an important determinant of lymph node metastasis. Nodal metastasis was found in 2.9% of patients with lesion thickness ≤5 mm and was 17.5% when tumor thickness was >5 mm. Their mean follow-up was 5.3 years.90
Depth of invasion. Tumors that reach Clark level V had a metastatic rate of 30.6%, whereas tumors that reach Clark level IV had only a 10% rate.15 A more recent study found that patients with nodal metastasis were significantly more likely to have invasion beyond Clark III.88 Furthermore, invasion beyond subcutaneous tissues has been found to be an independent indicator of outcome.87
Degree of differentiation.88,90,91 SCC lesions of poor histologic differentiation have double the local recurrence rate and triple the metastatic rate of those lesion that are well differentiated.86 Others have also been unable to make a correlation.15,47,85
Immunosuppression. In the setting of organ transplantation, immunosuppression has been linked with development of nonmelanoma skin cancers, especially SCC.85,92 Although immunosuppressed patients may only account for 5% of the cutaneous SCC population,40 the overall risk to these patients of developing a malignancy is high. By one estimate, the chance of a patient developing one cutaneous malignancy post renal transplant is 66% within 24 years.93 In a series of 68 organ transplant patients with metastatic skin cancer (85% SCC), the median primary size was only 12 mm, the median depth of invasion was only 3.2 mm, and the 3-year disease-specific survival was only 56%, thus indicating a lower threshold for metastatic spread and worse prognosis in this clinical setting.94
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree