Dysphagia/Speech Rehabilitation
Jerilyn A. Logemann
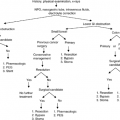
The speech-language pathologist is the usual professional to evaluate and treat speech and swallowing disorders at all points in a patient’s care whether at the time of their initial diagnosis or in palliative care (1). There are several individuals with speech and swallowing problems who are frequently cared for in palliative care by speech-language pathologists. These include patients who have been treated for head and neck cancer and the patients with degenerative neurologic disease. Patients with degenerative neurologic disease most often are those with Parkinson’s disease or with motor neuron disease, particularly amyotrophic lateral sclerosis (ALS). Each of these patient types exhibit different problems in their speech and swallowing and requires a different approach to their speech and swallowing management. There is no single remediation technique that can apply to all patients as the following three patients illustrate. Each of these patient types exhibit different speech, voice, and/or swallowing disorders requiring careful assessment and management.
A 56-year-old patient who had undergone chemoradiation for squamous cell carcinoma of the tongue base was treated for postoperative swallowing disorders but did not improve despite intensive swallowing therapy, practice, and his high motivation. After 6 months of living without oral feeding and dealing with chronic aspiration, the patient requested a total laryngectomy. The patient was counseled that after chemoradiation there were many factors that might make it difficult for him to eat well even after a total laryngectomy, which would, however, eliminate aspiration (2). When asked what he meant by “eating,” the patient stated that he wanted to take his nutrition by mouth, understanding that it would be unlikely that he could chew and swallow a steak or even mashed potatoes because of his previous chemoradiation. The chemoradiation made generating adequate pressure to push the food through his mouth a problem, especially through the reconstructed pharyngoesophagus. The patient stated that he understood this but did not want to continue nonoral feeding under any circumstance. He was also counseled about voice loss and alternative speech-rehabilitation methods were discussed. After the counseling, the total laryngectomy was completed at the patient’s request and the patient returned to full oral intake without meat or other foods that were difficult to chew and swallow. He was able to take soft foods and liquids of all kinds without any aspiration. To this patient communication was less important than eating, but he received a surgical voice restoration procedure, the tracheoesophageal puncture, and was able to communicate effectively.
The patient developed a recurrence in the pharynx and when in palliative care, continued to be able to speak but needed a diet of thinner and thinner foods, as he was unable to generate adequate pressure to swallow anything thicker. Regular swallow reevaluation by the speech-language pathologist, as the patient’s function deteriorated, resulted in continued oral feeding until his death.
Another patient after having had a surgical procedure to remove a squamous cell cancer at the base of his tongue with deltopectoral flap reconstruction was referred by his medical oncologist. The closure pulled his remaining oral tongue backward so that it reduced his ability to articulate speech sounds produced at the front of the mouth with his anterior tongue. He also complained that he had followed his surgeon’s suggestion to try to swallow mashed potatoes, but after 6 months of trying, he still could not eat by mouth and had a percutaneous endoscopic gastrostomy. After in-depth assessment of speech and swallowing, he was enrolled in active speech and swallowing therapy to improve range of oral tongue movement for speech and range of motion for the remaining tongue base for swallowing as well as therapy to improve his laryngeal elevation during swallowing, which was impaired from scar tissue. The patient was highly motivated and after 4 months of therapy, returned to oral intake, including mashed potatoes, as well as exhibited much improved articulation and speech understandability. Four years later, the patient developed a new tumor in the supraglottic larynx, which could not be resected and was treated with irradiation for palliation. After 6 months, the patient entered palliative care. His speech understandability further reduced because of decreased tongue mobility and hoarseness. His swallowing safety actually improved because his supraglottic tumor narrowed his airway and eliminated aspiration. He had a tracheostomy. As speech intelligibility worsened, various alternative augmentative communication strategies were introduced to allow the patient to communicate effectively with family, friends, and staff.
A third patient, with motor neuron disease, was losing all speech intelligibility and swallowing was worsening. The speech-language pathologist evaluated the patient for a computerized augmentative communication device and found one appropriate for the patient’s function of arms and hands. Swallowing was regularly reevaluated to identify safe food consistencies until the time that nonoral feeding was needed. These three patients illustrate the role of the speech-language pathologist in palliative care.
The speech-language pathologist generally approaches the patient in palliative care in the same way as s/he approaches the patient in rehabilitation, beginning by establishing goals to improve or maintain safe and efficient swallow and
understandable speech or communication in patients with significant medical problems such as, terminal squamous cell cancer of the head and neck or neurologic disease.
understandable speech or communication in patients with significant medical problems such as, terminal squamous cell cancer of the head and neck or neurologic disease.
Process for Palliative Care by the Speech-Language Pathologist
Although there is no single “best-swallow technique” or “best choice” of communication procedures or aids for all patients, there is a common process used to define the best swallow or speech/communication techniques for each individual patient. The process includes the following:
Counseling to allow the patient and family to understand the nature of the speech and swallowing problems the patient exhibits and the types of help that can be provided to them
Regular reevaluation of function to define changes in functional needs
Regular interaction/therapy as needed
Factors Determining Functional Needs in Swallowing and Communication
There are a number of factors that determine the patient’s functional needs in palliative care. These include the etiology and nature of the patient’s dysfunction(s), the patient’s and family’s reaction to the idea of therapy/intervention, and the patient’s goals for their function. As will be described in further detail later in this chapter, various medical diagnoses and the patient’s stage of deterioration result in various speech and swallowing problems that must be managed. In the case of chemoradiation, the patient’s exact radiation dose, area radiated, and the type of drugs used must be defined and it must be ascertained whether the chemoradiation was concurrent. For surgical procedures, both the extent and location of the resection, the nature of the surgical reconstruction, and location of recurrence of new tumor play major roles in defining the patient’s speech and swallowing abilities. Knowledge of the diagnosis is critical to appropriate speech and swallowing management for patients with neurologic disease. Patients with some diagnoses such as Parkinson’s disease benefit from active exercise while others such as those with motor neuron disease will worsen with any active exercise.
Parkinson’s Disease
The patient with Parkinson’s disease often exhibits worsening speech throughout his/her disease progression, which is usually quite slow and frequently lasts for at least twenty or more years. As the patient begins to have more difficulty being understood, they may benefit from a communication device, which ranges from a communication board, enabling them to point to words or letters to spell words, to a computerized system that can be highly sophisticated. If the patient develops dementia, use of some of these instruments may not be possible.
Motor Neuron Disease
The patient with motor neuron disease, most often ALS, may use oral speech for a period of a year or more and then generally needs some type of more sophisticated communication device. All patients with motor speech disorders require full assessment of their ability to use hand manipulations, typing, and other types of motor movements to control the various devices. There are patients with a diagnosis of ALS who use computerized artificial communication systems for years prior to their death.
Partial Laryngectomy
A partial laryngectomy for cancer of the larynx, either a supraglottic (horizontal partial laryngectomy) or hemilaryngectomy (vertical partial laryngectomy) generally causes some change in voice (hoarseness), as well as potential difficulty in protecting the airway during swallowing (3, 4, 5, and sometimes unremitting aspiration. There are a number of rehabilitation procedures involving volitional airway protection for swallowing, which patients can be taught, as well as exercises to improve range of motion of residual structures in the larynx (6, 7, 8).
Total Laryngectomy
The patient who receives a total laryngectomy will obviously have no voice source any longer and will need to replace that with either an artificial larynx, esophageal speech, or tracheoesophageal puncture (surgical prosthetic) voice restoration (9, 10, 11, 12, 13, 14). The latter procedure has become quite popular, as it restores voice rather quickly and the patient does not need to go through the long process of learning esophageal speech. However, to be a good candidate for a tracheoesophageal puncture, the patient must be willing to maintain a small prosthesis in the puncture site and therefore to do more stomal care. If these patients develop a recurrence of their disease, the prosthesis may need to be removed and they may need to use an artificial larynx.
Total laryngectomy also creates changes in swallowing, requiring the patient to increase the effort and pressure needed to swallow postoperatively (6, 15, 16, 17). However, after total laryngectomy the patient should be able to eat a full, normal diet. Few patients experience more significant swallowing problems related to a stricture or narrowing in their reconstructed pharyngoesophagus or a flap of “extra” mucosa at the base of the tongue known as a pseudoepiglottis (6). With recurrence of disease these problems, particularly cervical esophageal strictures, may recur or worsen.
The involvement of the speech-language pathologist in palliative care is a relatively new development but is increasing with some rapidity. In care of patients who are terminally ill, it is important that a team approach be used involving the speech-language pathologist with the gastroenterologist in management of oropharyngeal (speech-language pathologist) and esophageal (gastroenterologist) disorders. In addition, the interaction of speech-language pathologists with the patient’s primary care physician and others can facilitate smooth transitions for the patients as their functions may worsen. The constant goal is always to maintain optimal function for the patient in terms of communication with staff and family as well as best nutritional intake.
High-Dose Chemoradiation
Concomitant high-dose chemotherapy and radiation therapy to the head and neck are often called organ preservation protocols. They are designed to preserve the anatomic continuity of the upper aerodigestive tract by curing the patient’s disease without the need for surgery and at the same time maintaining function. Recent studies have shown,
however, that for some patients, some of the functions of the upper aerodigestive tract are not maintained in these protocols, particularly swallowing ability (18). To date, the particular high-dose chemoradiation protocols that have the greatest swallowing toxicity and the tumor locations where greatest toxicity is seen because of these protocols are just beginning to be defined (19). Currently, it appears that the patient with a hypopharyngeal tumor is at the greatest risk. The swallowing disorders of these individuals often are severe and prolonged, and are sometimes permanent. They include severely restricted laryngeal elevation and often virtually absent pharyngeal wall contraction. Reduced opening of the upper esophageal sphincter is a result of both of these problems. During swallowing, there is little pressure generated on the food to drive it through the pharynx and into the esophagus, leaving most of the food in the pharynx to be aspirated after the swallow. Some patients also develop cervical esophageal strictures, which require repeated dilatation, where possible. Some of these patients require conversion to total laryngectomy in an attempt to eat. However, such a conversion may not result in successful return to full oral intake, because total laryngectomy requires generation of even more pressure to drive the bolus through the reconstructed pharyngoesophagus than does normal swallowing. Because these patients already have diminished ability to generate pressure to drive food through the pharynx and, in this case, the pharyngoesophagus, a total laryngectomy will stop chronic aspiration of the patient’s own secretions and of food and liquid but may not enable the patient to get adequate nutrition orally.
however, that for some patients, some of the functions of the upper aerodigestive tract are not maintained in these protocols, particularly swallowing ability (18). To date, the particular high-dose chemoradiation protocols that have the greatest swallowing toxicity and the tumor locations where greatest toxicity is seen because of these protocols are just beginning to be defined (19). Currently, it appears that the patient with a hypopharyngeal tumor is at the greatest risk. The swallowing disorders of these individuals often are severe and prolonged, and are sometimes permanent. They include severely restricted laryngeal elevation and often virtually absent pharyngeal wall contraction. Reduced opening of the upper esophageal sphincter is a result of both of these problems. During swallowing, there is little pressure generated on the food to drive it through the pharynx and into the esophagus, leaving most of the food in the pharynx to be aspirated after the swallow. Some patients also develop cervical esophageal strictures, which require repeated dilatation, where possible. Some of these patients require conversion to total laryngectomy in an attempt to eat. However, such a conversion may not result in successful return to full oral intake, because total laryngectomy requires generation of even more pressure to drive the bolus through the reconstructed pharyngoesophagus than does normal swallowing. Because these patients already have diminished ability to generate pressure to drive food through the pharynx and, in this case, the pharyngoesophagus, a total laryngectomy will stop chronic aspiration of the patient’s own secretions and of food and liquid but may not enable the patient to get adequate nutrition orally.
These swallowing impairments are thought to result from severe fibrosis, particularly in the muscles of the pharynx, which appear to be quite sensitive to radiotherapy. In some cases, this fibrosis continues to worsen over time so that immediately after the completion of their radiotherapy the patient may be able to continue to eat successfully, but a year or two later may be unable to swallow efficiently and safely. If the larynx is in the field of radiotherapy, changes in voice quality may result, most of which are relatively temporary. Ability to articulate speech sounds is relatively unimpaired compared with swallowing function. With any disease recurrence these problems may worsen significantly and a nonoral feeding may be required.
Table 12.1 Postural Techniques Generally Most Appropriate For Each Swallow Disorder and the Physiologic/Anatomic Effect(s) of the Posture on Pharyngeal Dimensions or Bolus Flow | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Management of Tumors of the Hard and/or Soft Palate
Generally, the patient who has a tumor of the hard palate, which will be surgically removed, should be seen preoperatively by the maxillofacial prosthodontist and is frequently provided an intraoral obturator prosthesis by the maxillofacial prosthodontist at the time of surgery. In that way, when the patient awakens after surgery, they have a temporary prosthesis in place (20, 21). This prosthesis is then redesigned once the patient’s healing is complete, at 2 to 4 or more weeks postoperatively. With this temporary prosthesis in place, the patient’s speech and swallowing are often relatively intact.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree