Fig. 15.1
Van Nuys DCIS Classification. DCIS patients are separated in high nuclear grade (grade 3) and non-high nuclear grade (grades 1 and 2). Non-high nuclear grade cases are then separated by the presence or absence of necrosis. Lesions in group 3 (high nuclear grade) may or may not show necrosis
Nuclear grade is scored by previously described methods [32, 38, 40]. Essentially, low-grade nuclei (grade 1) are defined as nuclei 1–1.5 red blood cells in diameter with diffuse chromatin and unapparent nucleoli. Intermediate nuclei (grade 2) are defined as nuclei 1–2 red blood cells in diameter with coarse chromatin and infrequent nucleoli. High-grade nuclei (grade 3) are defined as nuclei with a diameter greater than two red blood cells, with vesicular chromatin, and one or more nucleoli. Mitotic activity is usually identifiable in high grade DCIS, but infrequently in lower grades I and II.
In the Van Nuys classification, no requirement is made for a minimum or specific amount of high nuclear grade DCIS, nor is any requirement made for a minimum amount of comedo-type necrosis. Occasional desquamated or individually necrotic cells are ignored and are not scored as comedo-type necrosis.
The most difficult part of most classifications is nuclear grading, particularly the intermediate grade lesions. The subtleties of the intermediate grade lesion are not important to the Van Nuys classification; only nuclear grade 3 need be recognized. The cells must be large and pleomorphic, lack architectural differentiation and polarity, have prominent nucleoli and coarse clumped chromatin, and generally show mitoses [32, 40, 44].
The Van Nuys classification is useful because it divides DCIS into three different biologic groups with different risks of local recurrence after breast conservation therapy (Fig. 15.2). This pathologic classification, when combined with tumor size, age, and margin status, is an integral part of the USC/Van Nuys Prognostic Index (USC/VNPI), a system that will be discussed in detail.
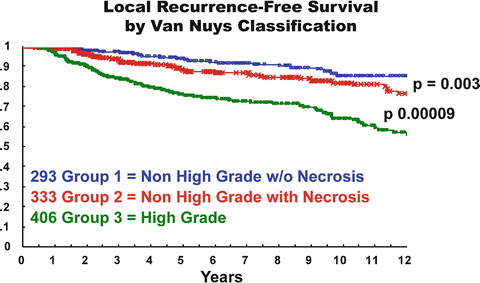

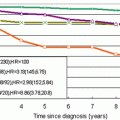
Fig. 15.2
Probability of local recurrence-free survival for 1,046 breast conservation patients using Van Nuys DCIS pathologic classification
Progression to Invasive Breast Cancer
Which DCIS lesions will become invasive and when will that happen? These are the most important questions in the DCIS field today. Currently, there is intense molecular biologic study regarding the progression of genetic changes in normal breast epithelium to DCIS and then to invasive breast cancer. Most of the genetic and epigenetic changes present in invasive breast cancer are already present in DCIS. To date, no genes uniquely associated with invasive cancer have been identified. As DCIS progresses to invasive breast cancer, quantitative changes in the expression of genes related to angiogenesis, adhesion, cell motility, and the composition of the extracellular-matrix may occur [2, 16].
Immunohistochemical and Molecular Phenotypes in DCIS
It has been recognized for some time that there is a substantial concordance between the grade of a DCIS and its associated invasive carcinoma, such that low grade lesions, regardless of the classification scheme used, are largely associated with lower grade invasive carcinomas while high grade DCIS are associated with higher grade invasive carcinomas. Additionally the frequency of specific biomarkers in DCIS varies with the grade of the lesion: estrogen and progesterone receptors are usually expressed in DCIS, but less so in high grade DCIS, while HER-2 and elevated proliferative markers such as Ki-67 are features of high grade DCIS. More recently surrogate molecular phenotypes defined by immunohistochemistry have identified DCIS phenotypes corresponding to luminal A, luminal B, Her-2 and triple negative/basal phenotypes in invasive breast cancer. Luminal A and B DCIS phenotypes are more frequent in the low = intermediate grade lesions, where HER-2, triple negative and basal phenotypes are more common among high grade DCIS [47, 48].
Currently gene expression profiling for invasive carcinomas can segregate carcinomas at significantly different risks of distant relapse (10 years) and indicate the likelihood of a benefit from chemotherapy. Hannemann et al. were able to segregate DCIS with and without invasive carcinomas by a group of 35 genes, and stratify DCIS by another 43 gene classifer into well differentiated vs. poorly differentiated (high grade) subtypes [49]. Additionally these authors confirmed the association of ER expression in low-intermediate DCIS and HER-2 in high grade DCIS.
Solin et al. presented a new DCIS gene signature RT-PCR assay which can separate out three risk groups based on their gene expression profiles [50]. The genes were selected from the existing Oncotype DX assay and the ECOG 5194 registration trial archived tissue was used for validation. The ECOG trial examined the frequencies of local recurrences in DCIS patients treated by excision alone with minimum 3 mm margins. Low to intermediate grade DCIS had to be 25 mm or less in greatest extent, while high grade DCIS could not exceed 10 mm.
All patients entered into the trial had to have specimens examined by a serial sequential tissue protocol which permits reproducible determination of size (extent), margin widths and excludes microinvasive foci. Cases which did not meet these criteria were excluded. Specimens examined by less rigorous means cannot be reliably evaluated by the assay, e.g., the DCIS may exceed the size limit or exhibit margins which are suboptimal and the RS reported may not be valid for the case submitted. By way of contrast two recent nomograms designed to predict recurrence following breast conservation therapy for DCIS were based on archival materials which were not processed in the serial sequential method and for which data on size and margins was frequently missing. Kerlikowski et al. evaluated specimens which were generated from many institutions without a common protocol, and without accurately determined size or margin status [51]. Rudloff et al. developed a nomogram which was obtained from a single institution but without a single protocol and not examined by the serial subgross technique [52].
The ECOG study dichotomized DCIS into low intermediate vs. high grade and only required a 3 mm margin. It will remain to be seen whether reanalysis of the pathology data will permit greater separation of subsets of DCIS comparable to what can be achieved in the USC/VNPI.
Because most patients with DCIS had been treated with mastectomy, knowledge of the natural history of this disease is relatively recent. The studies of Page et al. [53, 54] and Rosen et al. [55] provide information regarding the outcome of low grade DCIS without treatment. In these studies, patients with noncomedo DCIS, the majority, incidental foci in diagnostic excision biopsies for palpable disease, were initially misdiagnosed as benign lesions and therefore went untreated, aside from initial biopsy. Subsequently, approximately 25–35 % of these patients developed invasive breast cancer, generally within 10 years [53, 54]. Had the lesions been high-grade comedo DCIS, the invasive breast cancer rate likely would have been higher than 35 % and the time to invasive recurrence shorter. With few exceptions, in both of these studies, the invasive breast carcinoma was of the ductal type and located at the site of the original DCIS. These findings suggest that not all DCIS lesions progress to invasive breast cancer or become clinically significant [56]
Page and associates updated their series in 2002 and 2005 [53, 54, 57]. Of 28 women with low-grade DCIS misdiagnosed as benign lesions and treated only by biopsy between 1950 and 1968, 11 patients have recurred locally with invasive breast cancer (39 %). Eight patients developed recurrence within the first 12 years. The remaining three were diagnosed over 23–42 years. Five patients developed metastatic breast cancer (18 %) and died from the disease within 7 years of developing invasive breast cancer. These recurrence and mortality rates, at first glance, seem alarmingly high. However, they are only slightly worse than what can be expected with long-term follow-up of patients with lobular carcinoma in situ, a disease that most clinicians are willing to treat with careful clinical follow-up. In addition, these patients were treated with biopsy only. No attempt was made to excise these lesions with a clear surgical margin. The natural history of low-grade DCIS can extend over 40 years and is markedly different from that of high-grade DCIS.
Microinvasion
The incidence of microinvasion is difficult to quantitate because until 1997 there was no formal and universally accepted definition of exactly what constitutes microinvasion. The fifth edition of The Manual for Cancer Staging carried the first official definition of what is now classified as pT1mic and read as follows: “Microinvasion is the extension of cancer cells beyond the basement membrane into adjacent tissues with no focus more than 0.1 cm in greatest dimension. When there are multiple foci of microinvasion the size of only the largest focus is used to classify the microinvasion (do not use the sum of all individual foci). The presence of multiple foci of microinvasion should be noted, as it is with multiple larger invasive carcinomas.”
The reported incidence of occult invasion (invasive disease at mastectomy in patients with a biopsy diagnosis of DCIS) varies greatly, ranging from as little as 2 % to as much as 21 % [58]. This problem was addressed in the investigations of Lagios et al. [32, 38].
Lagios et al. performed a meticulous serial subgross dissection correlated with specimen radiography. Occult invasion was found in 13 of 111 mastectomy specimens from patients who had initially undergone excisional biopsy of DCIS. All occult invasive cancers were associated with DCIS greater than 45 mm in extent; the incidence of occult invasion approached 50 % for DCIS greater than 55 mm. In the study of Gump et al. [59], foci of occult invasion were found in 11 % of patients with palpable DCIS but in no patients with clinically occult DCIS. These results suggest a correlation between the size of the DCIS lesion and the incidence of occult invasion. Clearly, as the size of the DCIS lesion increases, microinvasion and occult invasion become more likely.
If even the smallest amount of invasion is found, the lesion should not be classified as DCIS. It is a T1mic (if the largest invasive component is 1 mm or less) with an extensive intraductal component (EIC). If the invasive component is 1.1 mm to 5 mm, it is a T1a lesion with EIC. If there is only a single focus of invasion, these patients do quite well. When there are many tiny foci of invasion, these patients have a poorer prognosis than expected [10]. Unfortunately, the TNM staging system does not have a T category that fully reflects the malignant potential of lesions with multiple foci of invasion since they are all classified by the largest single focus of invasion. De Mascarel et al. have noted that microinvasive foci consisting of single cells have no impact on outcome while those comprising cohesive groups of cells are associated with a demonstrable increase in distant recurrence and death [60].
Multicentricity and Multifocality of Ductal Carcinoma In Situ
Multicentricity is generally defined as DCIS in a quadrant other than the quadrant in which the original DCIS (index quadrant) was diagnosed. There must be normal breast tissue separating the two foci. However, definitions of multicentricity vary among investigators. Hence, the reported incidence of multicentricity also varies. Rates from 0 to 78 % [7, 55, 61, 62], averaging about 30 %, have been reported. Twenty-five years ago, the 30 % average rate of multicentricity was used by surgeons as the rationale for mastectomy in patients with DCIS.
Holland et al. [63] evaluated 82 mastectomy specimens by taking a whole-organ section every 5 mm. Each section was radiographed. Paraffin blocks were made from every radiographically suspicious spot. In addition, an average of 25 blocks were taken from the quadrant containing the index cancer; random samples were taken from all other quadrants, the central subareolar area, and the nipple. The microscopic extension of each lesion was verified on the radiographs. This technique permitted a three-dimensional reconstruction of each lesion. This study demonstrated that most DCIS lesions were larger than expected (50 % were greater than 50 mm), involved more than one quadrant by continuous extension (23 %), but most importantly, were unicentric (98.8 %). Only one of 82 mastectomy specimens (1.2 %) had “true” multicentric distribution with a separate lesion in a different quadrant. This study suggests that complete excision of a DCIS lesion is possible due to unicentricity but may be extremely difficult due to larger than expected size. In a recent update, Holland reported whole-organ studies in 119 patients, 118 of whom had unicentric disease [64]. This information, when combined with the fact that most local recurrences are at or near the original DCIS, suggests that the problem of multicentricity per se is not important in the DCIS treatment decision-making process.
Multifocality is defined as separate foci of DCIS within the same ductal system. The studies of Holland et al. [63, 64] and Noguchi et al. [65] suggest that a great deal of multifocality may be artifactual, resulting from looking at a three-dimensional arborizing entity in two dimensions on a glass slide. It would be analogous to saying that the branches of a tree were not connected if the branches were cut at one plane, placed separately on a slide, and viewed in cross-section [53]. Multifocality may be due to small gaps of DCIS or skip areas within ducts as described by Faverly et al. [43]. Multifocality is more easity recognized when a serial sequential tissue processing technique as opposed to random sampling is employed.
Detection and Diagnosis
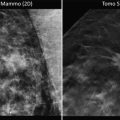
The importance of quality mammography cannot be overemphasized. Currently, most patients with DCIS (more than 90 %) present with a nonpalpable lesion detected by mammography. The most common mammographic finding is microcalcification, frequently clustered and generally without an associated soft-tissue abnormality. More than 80 % of DCIS patients exhibit microcalcifications on preoperative mammography. The patterns of these microcalcifications may be focal, diffuse, or ductal, with variable size and shape. Patients with comedo DCIS tend to have “casting calcifications.” These are linear, branching, and bizarre and are almost pathognomonic for comedo DCIS [66] (Fig. 15.3). It is important to note that many DCIS with prominent comedonecrosis fail to exhibit mammographic microcalcifications, and among others, microcalcification is seen only intermittently.
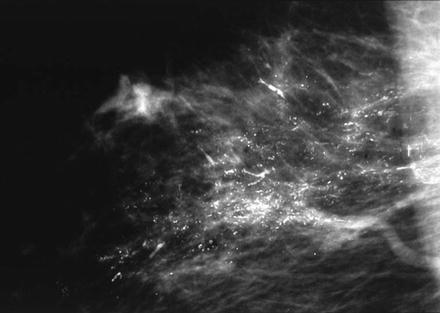

Fig. 15.3
Mediolateral mammography in a 43-year-old woman shows irregular branching calcifications. Histopathology showed high-grade comedo DCIS, Van Nuys group 3
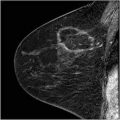
Thirty-two percent of noncomedo lesions in this series did not have mammographic calcifications, making them more difficult to find and the patients more difficult to follow, if treated conservatively. When noncomedo lesions are calcified, they tend to have fine granular powdery calcifications or crushed stone-like calcifications (Fig. 15.4).
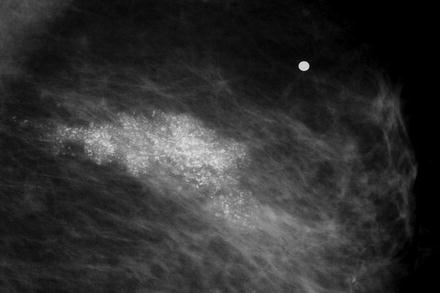

Fig. 15.4
Crushed stone type calcifications
A major problem confronting surgeons relates to the fact that calcifications do not always map out the entire DCIS lesion, particularly those of the noncomedo type. Even though all the calcifications are removed, in some cases, noncalcified DCIS may be left behind. Conversely, in some patients, the majority of the calcifications are benign and map out a lesion bigger than the true DCIS lesion. In other words, the DCIS lesion may be smaller, larger, or the same size as the calcifications that lead to its identification. Calcifications more accurately approximate the size of high-grade comedo lesions than low-grade noncomedo lesions [63].
Before mammography was common or of good quality, most DCIS was usually clinically apparent, diagnosed by palpation or inspection; it was gross disease. Gump et al. divided DCIS by method of diagnosis into gross and microscopic disease [59]. Similarly, Schwartz et al. divided DCIS into two groups: clinical and subclinical [33]. Both researchers thought patients presenting with a palpable mass, a nipple discharge, or Paget’s disease of the nipple required more aggressive treatment. Schwartz believed that palpable DCIS should be treated as though it were an invasive lesion. He suggested that the pathologist simply has not found the area of invasion. Although it makes perfect sense to believe that the change from nonpalpable to palpable disease is a poor prognostic sign, our group has not been able to demonstrate this for DCIS. In our series, when equivalent patients (by size and nuclear grade) with palpable and nonpalpable DCIS were compared, they did not differ in the rate of local recurrence or mortality.
If a patient’s mammogram shows an abnormality, most likely, it will be microcalcifications, but it could be a nonpalpable mass or an architectural distortion. At this point, additional radiologic workup needs to be performed. This generally includes compression mammography and magnification views. Ultrasonography should be performed on all calcifications that require biopsy to rule out the presence of a mass that can be biopsied with ultrasound guidance. Magnetic resonance imaging (MRI) has become increasingly popular and if often used to map out the size and shape of biopsy proven DCIS lesions or invasive breast cancers and to rule out other foci of multifocal, multicentric or contralateral cancer.
Biopsy and Tissue Handling
If radiologic work-up shows an occult lesion that requires biopsy, there are multiple approaches: fine-needle aspiration biopsy (FNAB), core biopsy (with various types and sizes of needles), and directed surgical biopsy using guide wires or radioactive localization. FNAB is generally of little help for nonpalpable DCIS. With FNAB, it is possible to obtain cancer cells, but because there is insufficient tissue, there is no architecture. So, although the cytopathologist can say that malignant cells are present, the cytopathologist cannot say whether or not the lesion is invasive.
Stereotactic core biopsy became available in the early 1990s, and is now widely used. Dedicated digital tables make this a precise tool. Currently large gauge vacuum assisted needles are the tools of choice for diagnosing DCIS. Ultrasound guided biopsy also became popular in the 1990s but is of less value for DCIS since most DCIS lesions do not present with a mass that can be visualized by ultrasound. All suspicious microcalcifications should be evaluated by ultrasound since a mass will be found in 5–15 % [9].
Open surgical biopsy should only be used if the lesion cannot be biopsied using minimally invasive techniques. This should be a rare event with current image-guided biopsy techniques and occur in less than 5 % of cases [9, 67]. Currently, at Hoag Memorial Hospital Presbyterian, open biopsy is performed for diagnostic purposes in only 1 % of patients and we treat more than 700 new breast cancer patients per year. Needle localization segmental resection should be a critical part of the treatment regimen not the diagnosis [67].
Whenever needle localization excision is performed, whether for diagnosis or treatment, intraoperative specimen radiography and correlation with the preoperative mammogram are mandatory. Margins should be inked or dyed and specimens should be serially sectioned and a second X-ray of the slices should be obtained. The tissue sections should be arranged and processed in sequence. Pathologic reporting should include a description of all architectural subtypes, a determination of nuclear grade, an assessment of the presence or absence of necrosis, the measured extent of the lesion, and the margin status with measurement of all margins and, in particular, the closest margin [68–70].
Tumor size should be determined by direct measurement or ocular micrometry from stained slides for smaller lesions. For larger lesions, a combination of direct measurement and estimation, based on the distribution of the lesion in a sequential series of slides, should be used. The proximity of DCIS to an inked margin should be determined by direct measurement or ocular micrometry. The closest single distance between any involved duct containing DCIS and an inked margin should be reported.
If the lesion is large and the diagnosis unproven, either stereotactic or ultrasound-guided vacuum-assisted biopsy should be the first step. If the patient is motivated for breast conservation, a multiple-wire-directed oncoplastic excision can be planned. This will give the patient her best chance at two opposing goals: clear margins and good cosmesis. The best chance at completely removing a large lesion is with a large initial excision. The best chance at good cosmesis is with a small initial excision. It is the surgeon’s job to optimize these opposing goals. A large quadrant resection should not be performed unless there is histologic proof of malignancy. This type of resection may lead to breast deformity, and should the diagnosis prove to be benign, the patient will have undergone needless surgery.
Removal of nonpalpable lesions is best performed by an integrated team of surgeon, radiologist, and pathologist. The radiologist who places the wires and interprets the specimen radiograph must be experienced, as must the surgeon who removes the lesion, and the pathologist who processes the tissue.
Treatment
For most patients with DCIS, there will be no single correct treatment. There will generally be a choice. The choices, although seemingly simple, are not. As the choices increase and become more complicated, frustration increases for both the patient and her physician [71, 72].
Counseling the Patient with Biopsy-Proven Ductal Carcinoma In Situ
It is never easy to tell a patient that she has breast cancer. But is DCIS really cancer? From a biologic point of view, DCIS is unequivocally cancer. But when we think of cancer, we generally think of a disease that, if untreated, runs an inexorable course toward death. That is certainly not the case with DCIS [54]. We must emphasize to the patient that she has an incomplete cancerous lesion, a preinvasive lesion, which at this time is not a threat to her life. In our series of 1,585 patients with DCIS, the breast cancer specific mortality rate is 0.5 % at 12-years. Numerous other DCIS series [73–78] confirm an extremely low mortality rate.
Patients often ask why there is any mortality rate at all if DCIS is truly a noninvasive lesion. If DCIS recurs as an invasive lesion and the patient goes on to die from metastatic breast cancer, the source of the metastases is clear, the invasive disease. But what about the patient who undergoes mastectomy, only DCIS is found, and sometime later develops metastatic disease or a patient who is treated with breast preservation who never develops a local invasive recurrence but still dies of metastatic breast cancer? The latter patients probably had an invasive focus with established metastases at the time of their original treatment but the invasive focus was missed during routine histopathologic evaluation. Routine examination of mastectomy material is inadequate for patients with DCIS. A more thorough and methodical approach utilizing specimen radiography is required. No matter how carefully and thoroughly a specimen in examined, it is still a sampling process and a small focus of invasion can be missed.
One of the most frequent concerns expressed by patients once a diagnosis of cancer has been made is the fear that the cancer has spread. We are able to assure patients with DCIS that no invasion was seen microscopically and the likelihood of systemic spread is minimal.
The patient needs to be educated that the term “breast cancer” encompasses a wide range of lesions of varying degrees of aggressiveness and lethal potential. The patient with DCIS needs to be reassured that she has a minimal lesion and that she is likely going to need some additional treatment, which may include surgery, radiation therapy, an antiestrogen, or some combination of those modalities. She needs reassurance that she will not need chemotherapy, that her hair will not fall out, and that it is highly unlikely that she will die from this lesion. She will, of course, need careful clinical follow-up.
Endpoints for Patients with DCIS
When evaluating the results of treatment for patients with breast cancer, a variety of endpoints must be considered. Important endpoints include local recurrence (both invasive and DCIS), regional recurrence (such as the axilla), distant recurrence, breast cancer-specific survival, overall survival, and quality of life. The importance of each endpoint varies depending on whether the patient has DCIS or invasive breast cancer
When treating invasive cancer, the most important endpoints are distant recurrence and breast cancer-specific survival; in other words, living or dying from breast cancer. For invasive breast cancer, a variety of different systemic treatments have been shown to significantly improve survival. These include a wide range of chemotherapeutic regimens, endocrine treatments, immunologic therapies, and others. Variations in local treatment were incorrectly thought not to affect survival. [23, 79]. They do, however, affect local recurrence and local invasive recurrence affect survival. Meta-analyses have shown that for every four local recurrences prevented, one breast cancer death is also prevented [80, 81]
DCIS is similar to invasive breast cancer in that variations in local treatment affect local recurrence, but no study to date has shown a significant difference in distant disease-free or breast cancer-specific survival, regardless of any treatment (systemic or local) and no study is likely to show a difference since there are so few breast cancer deaths in patients with pure DCIS. The most important outcome measure, breast cancer-specific survival, is essentially the same no matter what local or systemic treatment is given. Consequently, local recurrence has become the most commonly used endpoint when evaluating treatment for patients with DCIS.
A meta-analysis of four randomized DCIS trials comparing excision plus radiation therapy versus excision alone was published in 2007 [77]. It contained 3,665 patients. Radiation therapy decreased local recurrence by a statistically significant 60 % but overall survival was slightly (but statistically insignificantly) worse in the radiotherapy group with a relative risk of 1.08 These data are dissimilar to those of the Early Breast Cancer Trialists’ Collaborative Group and deserve further analysis [80, 81]. Half of the recurrences in the meta-analysis were DCIS and could not possibly affect survival. Of the remaining invasive recurrences, 80–90 % are cured by early detection and treatment. This should result in a slight trend toward a lower survival for the excision alone group just as would be expected from the Early Breast Cancer Trialists’ Collaborative Group. But exactly the opposite was seen, a nonsignificant trend toward a better survival in the excision only patients. The authors of the meta-analysis felt that with longer follow-up, the higher local recurrence rate for excision alone will likely result in a lower overall survival at some point in time. But for the time being, that has not happen and a small detrimental effect secondary to radiation therapy must be considered a possibility.
Local recurrences are clearly important to prevent in patients treated with DCIS. They are demoralizing. They often lead to mastectomy and if they are invasive, they upstage the patient and are a threat to life. But protecting DCIS patients from local recurrence must be balanced against the potential detrimental effects of the treatments given.
Following treatment for DCIS, 40–50 % of all local recurrences are invasive. About 10–20 % of DCIS patients who develop local invasive recurrences develop distant metastases and die from breast cancer [82, 83]. Long-term, this could translate into a mortality rate of about 0.5 % for patients treated with mastectomy, 1–2 % for conservatively treated patients who receive radiation therapy (if there is no mortality associated with radiation therapy) and 2–3 % for patients treated with excision alone. In order to save their breasts, many patients are willing to accept this theoretic, and as of now statistically unproven, small absolute risk associated with breast conservation therapy.
Treatment Options
Mastectomy
Mastectomy is, by far, the most effective treatment available for DCIS if our goal is simply to prevent local recurrence. Most mastectomy series reveal local recurrence rates of approximately 1 % with mortality rates close to 0 [84]. In our mastectomy series of 539 patients, none of whom received radiation therapy or tamoxifen, we have had 10 local recurrences (8 invasive and 2 DCIS). One of the patients with an invasive local recurrence developed metastatic disease. In addition, two other patients developed metastatic breast cancer without developing a local recurrence. The absolute rate of distant recurrence was 0.6 %.
But, mastectomy is an aggressive form of treatment for patients with DCIS. It clearly provides a local recurrence benefit but only a theoretical survival benefit. It is, therefore, often difficult to justify mastectomy, particularly for otherwise healthy women with screen-detected DCIS, during an era of increasing utilization of breast conservation for invasive breast carcinoma. Mastectomy is indicated in cases of true multicentricity (multi-quadrant disease) and when a unicentric DCIS lesion is too large to excise with clear margins and an acceptable cosmetic result.
Genetic positivity for one of the breast cancer associated genes (BRCA1, BRCA2) is not an absolute contraindication to breast preservation but many patients who are genetically positive and who develop DCIS seriously consider bilateral mastectomy and salpingo-oophorectomy.
Breast Conservation
The most recently available Surveillance Epidemiology and End Results (SEER) data reveal that 74 % of patients with DCIS are treated with breast conservation. While breast conservation is now widely accepted as the treatment of choice for DCIS, not all patients are good candidates. Certainly, there are patients with DCIS whose local recurrence rate with breast preservation is so high (based on factors that will be discussed later in this chapter) that mastectomy is clearly a more appropriate treatment. However, the majority of women with DCIS diagnosed currently are candidates for breast conservation. Clinical trials have shown that local excision and radiation therapy in patients with negative margins can provide excellent rates of local control [73, 76–78, 85–88]. However, even radiation therapy may be overly aggressive since many cases of DCIS may not recur or progress to invasive carcinoma when treated by excision alone [32, 54, 89–93].
Reasons to Consider Excision Alone
There are a number of lines of reasoning that suggest that excision alone may be an acceptable treatment for selected patients with DCIS.
1.
Common Usage: Excision alone is already common in spite of the randomized data that suggest that all conservatively treated patients benefit from radiation therapy. SEER Data reflect that excision alone is being used as complete treatment for DCIS in 35 % of all DCIS patients. American doctors and patients have embraced the concept of excision alone for DCIS.
2.
Anatomic: Evaluation of mastectomy specimens using the serial subgross tissue processing technique reveals that most DCIS is unicentric (involves a single breast segment and is radial in its distribution [39, 43, 63, 64, 94, 95]. Using the same technique and evaluating patients with small extent disease (≤25 mm) more clearly established that the majority of image detected DCIS can be adequately excised [32, 38]. This means that in many cases, it is possible to excise the entire lesion with a segment or quadrant resection. Since DCIS, by definition, is not invasive and has not metastasized, it can be thought of in Halstedian terms. Complete excision should cure the patient without any additional therapy. Holland and Faverly have shown that if 10 mm margins are achieved in all directions, the likelihood of residual DCIS is less than 10 % [43].
3.
Biologic: Some DCIS is simply not aggressive, for example small well-excised low-grade lesions bordering on atypical ductal hyperplasia. Lesions like this carry a low potential for development into an invasive lesion, about 1 % per year at most [53, 54, 57, 89, 96, 97]. This is only slightly more than lobular carcinoma in situ (LCIS), a lesion that is routinely treated with careful clinical follow-up.
4.
Pathology Errors: The differences between atypical ductal hyperplasia and low grade DCIS may be subtle. It is not uncommon for atypical ductal hyperplasia to be called DCIS. Such patients treated with excision and radiation therapy are indeed “cured of their DCIS”.
5.
Prospective Randomized Data: the prospective randomized DCIS trials show no difference in breast cancer-specific survival or overall survival, regardless of treatment after excision with or without breast irradiation [73, 76–78, 88]. If this is true, why not strive for the least aggressive treatment?
6.
Radiotherapy may do harm: Numerous studies have shown that radiation therapy for breast cancer may increase mortality from both lung cancer and cardiovascular disease [98–102]. Current radiotherapy techniques, which make use of CT planning, make every attempt to spare the heart and lungs from radiation exposure but long-term date are not available. If there is no proof that breast irradiation for patients with DCIS improves survival and there is proof that radiation therapy may cause harm, it makes perfect sense to spare patients from this potentially dangerous treatment whenever possible.
7.
Radiation therapy is expensive, time consuming, and is accompanied by significant side effects in a small percentage of patients (cardiac, pulmonary, etc.) [103]. Radiation fibrosis continues to occur but it is less common with current techniques than it was during the 1980s. Radiation fibrosis changes the texture of the breast and skin, makes mammographic follow-up more difficult, and may result in delayed diagnosis if there is a local recurrence. This will become much less of a problem if intraoperative radiation therapy (IORT) becomes a proven modality for DCIS [104].
8.
Some series show that there are more invasive recurrences in irradiated patients than in nonirradiated patients. In our own series, 39 % of excision only patients who recurred, recurred with invasive disease whereas 56 % of irradiated patients who recurred, recurred with invasive disease (p < 0.01). This is true in the series of Schwartz et al. [92] and Wong et al. [105]. In our series, the median time to recur after excision alone was 23 months while after excision and irradiation, it was 58 months (p < 0.01). This delay in the diagnosis of recurrence may contribute to the increased rate of local invasive recurrence in irradiated patients.
9.
If radiation therapy is given for the initial DCIS, it cannot be given again, at a later time, if there is a small invasive recurrence. In general, in favorable patients, we prefer to withhold radiation therapy initially and only give it to the few that ultimately recur with invasive disease. The use of radiation therapy with its accompanying skin and vascular changes make skin-sparing mastectomy, if needed in the future, more difficult to perform.
10.
Using commonly available histopathologic parameters, we can do better than the gold standard for local recurrence established by the prospective randomized trials. The “gold standard” for irradiated patients is a 16 % local recurrence rate at 12 years. This was established by the NSABP B-17 Trial [73, 85–87, 106]. Using tools such as the USC/Van Nuys Prognostic Index, it is possible to select patients with low scores ranging from 4 to 6. These patients recur at a rate of 6 % or less at 12 years without radiation therapy.
11.
Finally, within the 2008 NCCN (National Comprehensive Cancer Network) Guidelines, excision without radiation therapy (excision alone) has been added as an acceptable treatment for selected DCIS patients with low risk of recurrence [107]. Excision alone is now accepted and mainstream for favorable patients with DCIS
Distant Disease and Death
When a patient with DCIS, previously treated by any modality, develops a local invasive recurrence, followed by distant disease and death due to breast cancer, this stepwise progression makes sense. The patient has been upstaged by her local invasive recurrence. The invasive recurrence becomes the source of metastatic disease and death is now a possibility.
In contrast, when a previously treated patient with DCIS develops distant disease and there has been no invasive local recurrence, a completely different sequence of events must be postulated. This sequence implies that invasive disease was present within the original lesion but was never discovered and was already metastatic at the time of the original diagnosis. The best way to avoid missing an invasive cancer is with complete sequential tissue processing at the time the original lesion is treated. Nevertheless, even the most extensive evaluation may miss a small focus of invasion.
If, during histopathologic evaluation, even the tiniest invasive component is found, this patient can no longer be classified as having DCIS. She has invasive breast cancer and she needs to be treated as such. She will need sentinel node biopsy, radiation therapy if treated conservatively, and appropriate medical oncologic consultation and aftercare.
The Prospective Randomized Trials
All of the prospective randomized trials have shown a significant reduction in local recurrence for patients treated with radiation therapy compared with excision alone but no trial has reported a survival benefit, regardless of treatment [73, 76, 77, 85–88, 106, 108–111].
Only one trial has compared mastectomy with breast conservation for patients with DCIS and the data were only incidentally accrued. The National Surgical Adjuvant Breast Project (NSABP) performed protocol B-06, a prospective randomized trial for patients with invasive breast cancer [61, 112]. There were three treatment arms: total mastectomy, excision of the tumor plus radiation therapy, and excision alone. Axillary nodes were removed regardless of the treatment assignment.
During central slide review, a subgroup of 78 patients was confirmed to have pure DCIS without any evidence of invasion [61]. After 83 months of follow-up, the percent of patients with local recurrences were as follows: zero for mastectomy, 7 % for excision plus radiation therapy, and 43 % for excision alone [113]. In spite of these large differences in the rate of local recurrence for each different treatment, there was no difference among the three treatment groups in breast cancer-specific survival.
Contrary to the lack of trials comparing mastectomy with breast conservation, a number of prospective randomized trials comparing excision plus radiation therapy with excision alone for patients with DCIS have been performed: the NSABP (protocol B-17) [85], the European Organization for Research and Treatment of Cancer (EORTC), protocol 10,853 [88], the United Kingdom, Australia, New Zealand DCIS Trial (UK Trial) [76], and the Swedish Trial [78].
The results of NSABP B-17 were updated in 1995 [109], 1998; [87], 1999 [86], 2001 [73] and 2011 [106]. In this study, more than 800 patients with DCIS excised with clear surgical margins were randomized into two groups: excision alone versus excision plus radiation therapy. The main endpoint of the study was local recurrence, invasive or noninvasive (DCIS). The definition of a clear margin was non-transection of the DCIS.
After 15 years of follow-up, there was a statistically significant, 50 % decrease in local recurrence of both DCIS and invasive breast cancer in patients treated with radiation therapy. The overall local recurrence rate for patients treated by excision alone was 35 % at 15 years. For patients treated with excision plus breast irradiation, it was 19.8 %, a relative benefit of 43 % [106]. There was no difference in distant disease-free or overall survival in either arm. These data led the NSABP to confirm their 1993 position and to continue to recommend postoperative radiation therapy for all patients with DCIS who chose to save their breasts. This recommendation was clearly based primarily on the decreased local recurrence rate for those treated with radiation therapy and secondarily on the potential survival advantage it might confer.
The early results of B-17, in favor of radiation therapy for patients with DCIS, led the NSABP to perform protocol B-24 [86]. In this trial, more than 1,800 patients with DCIS were treated with excision and radiation therapy, and then randomized to receive either tamoxifen or placebo. After 15 years of follow-up, 16.6 % of patients treated with placebo had recurred locally, whereas, only 13.2 % of those treated with tamoxifen had recurred [106]. The difference, while small, was statistically significant for invasive local recurrence but not for noninvasive (DCIS) recurrence. A recent analysis of the subset of B24 patients known to be estrogen receptor-positive has shown no significant difference in ipsilateral in situ or invasive recurrence [114]. Again, there was no difference in distant disease-free or overall survival in either arm of the B-24 Trial.
The EORTC results were published in 2000 [88, 108]. This study was essentially identical to B-17 in design and margin definition. More than 1,000 patients were included. The data were updated in 2006 [110]. After 10 years of follow-up, 15 % of patients treated with excision plus radiation therapy had recurred locally compared with 26 % of patients treated with excision alone, results similar to those obtained by the NSABP at the same point in their trial. As in the B-17 Trial, there was no difference in distant disease-free or overall survival in either arm of the EORTC Trial. In the initial report, there was a statistically significant increase in contralateral breast cancer in patients who were randomized to receive radiation therapy. This was not maintained when the data were updated.
The United Kingdom, Australia, New Zealand DCIS Trial (UK Trial) was published in 2003 [76] and updated in 2011 [111]. This trial, which involved more than 1,694 patients, performed a two by two study in which patients could be randomized into two separate trials within a trial. The patients and their doctors chose whether to be randomized in one or both studies. After excision with clear margins (same non-transection definition as the NSABP), patients were randomized to receive radiotherapy (yes or no) and/or to tamoxifen versus placebo. This yielded four subgroups: excision alone, excision plus radiation therapy, excision plus tamoxifen, and excision plus radiation therapy plus tamoxifen. After a median follow-up of 12.7 years, those who received radiation therapy obtained a statistically significant decrease in ipsilateral breast tumor recurrence similar in magnitude to the ones shown by the NSABP and EORTC. Tamoxifen significantly reduced the incidence of ipsilateral DCIS recurrences but not invasive recurrences. It reduced the incidence of new contralateral breast cancers in a magnitude similar to NSABP B-24. As with the NSABP and the EORTC, there was no difference in survival, regardless of treatment, in any arm of the UK DCIS trial.
The Swedish DCIS Trial randomized 1,067 patients into two groups: excision alone versus excision plus radiation therapy. 1,046 have been followed for a mean of 8 years. Microscopically clear margins were not mandatory. 22 % of patients had microscopically unknown or involved margins. The cumulative incidence of local recurrence at 10-years was 21.6 % for excision only and 10.3 % for excision plus radiation therapy. There were 15 distant metastases and breast cancer related deaths in the excision only arm and 18 in the excision plus radiation therapy (p = ns) [78, 115].
All of these trials support the same conclusions. They all show that radiation therapy decreases local recurrence by a relative 50 % and they all show no survival benefit, regardless of treatment. Two trials show a decrease in local recurrence and contralateral breast cancer attributable to tamoxifen.
In 2007, Viani et al. published a meta-analysis of the four prospective randomized DCIS trials comparing excision alone with excision plus radiation therapy [77]. Three thousand six hundred and sixty-five patients were available for analysis. Pooled data revealed a 60 % reduction of both invasive and DCIS recurrences (p < 0.00001) with the addition of radiation therapy. There was, however, no decrease in distant metastases in those who received radiation therapy nor was there any survival benefit. Patients with high-grade lesions and involved margins received the most benefit from radiation therapy.
Limitations of the Prospective Randomized Trials
The randomized trials were designed to answer a single broad question: does radiation therapy decrease local recurrence? They have accomplished that goal. All have clearly shown that, overall, radiation therapy decreases local recurrence, but they cannot identify in which subgroups the benefit is so small, that the patients can be safely treated with excision alone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree