Ductal carcinoma in situ (DCIS) is a preinvasive clinical diagnosis that lies in the continuum between epithelial atypia and invasive cancer. Although it has been recognized and treated for almost three decades, much remains controversial about this disease and its optimal treatment. The incidence of DCIS has increased significantly since it was first described, largely as a consequence of widespread mammographic screening. Accordingly the presentation pattern has changed from a predominantly palpable lesion to one whose first indication is that of an incidentally discovered mammographic abnormality on routine screening examination. Because DCIS is a preinvasive condition with heterogenous potential for invasion, there remains ongoing debate over whether the current treatment recommendations represent overtreatment of some DCIS which may have never resulted in clinical consequences during a patient’s lifetime. This chapter reviews the epidemiology, diagnosis, and treatment of DCIS as well as some future directions for clinical management of the disease.
The incidence of DCIS has increased over 500% in the last 30 years, with over 50,000 women diagnosed annually.1 Data collected by the National Cancer Institute’s Breast Cancer Surveillance Consortium (BCSC) estimates that one case of DCIS is detected for approximately every 1300 screening mammography examinations performed.2 The incidence rate varies by age, ranging from approximately 1 in every 1800 mammograms among women aged 40 to 49 years to 1 in every 935 mammograms among women aged 70 to 84 years. The overall prevalence of screen-detected breast cancers diagnosed as DCIS was significantly higher in the younger, compared to the older age group (28.2%: age 40 to 49; 16% to 20%: age 60 to 84 years). With current treatment options, breast cancer mortality from DCIS is less than 2% at 10 years. The primary objectives in treatment of DCIS are to prevent local recurrence of DCIS or progression to invasive carcinoma, and to maintain the high rates of disease-free survival.3
By diagnosing and treating this burden of DCIS, a commensurate reduction in the incidence of early invasive cancer would be expected; however, this has not been reflected in the observed incidence rates for breast cancer.4 Moreover, the prevalence of undiagnosed DCIS is substantial and may be seen in up to 14% of women in routine autopsy series, confirming that many patients with unrecognized DCIS die with the disease and not of it.5
The risk factors for DCIS do not differ from those for invasive breast cancer, and it is widely accepted that DCIS is a precursor lesion to invasive breast cancer. However not all DCIS may have the ability to progress to invasion.6 By examining tumors that contain both an invasive and noninvasive component, studies have demonstrated that the expression of tumor markers is highly similar in both components.7,8 This suggests that genomics likely influence grade, rather than invasiveness. Despite significant advances in our understanding of cancer genomics, the specific genes that play a role in invasion have yet to be identified, and we are likely to discover that DCIS is the end product of a series of complex interactions resulting from both endogenous and exogenous influences.
Prior to widespread national adoption of screening mammography in the 1980s and 1990s, DCIS was largely detected on physical examination. The earliest case series of DCIS in the medical literature described 112 cases of DCIS collected from 1960 to 1969 at Memorial Hospital in New York where 87% of cases were associated with a palpable mass or poorly defined thickening of the breast, and 25% were associated with pathologic nipple discharge with or without an underlying mass.9 Cases were diagnosed after surgical removal of a mass or central duct excision. In contrast, DCIS currently comprises 28% of all breast cancers diagnosed in the United States,10 with the majority of DCIS diagnosed on screening mammography in entirely asymptomatic women.
In approximately 80% of cases, DCIS is detected by microcalcifications on mammography.11 The remainder of cases are detected as soft tissue mass or distortion (10%) or a combination of microcalcifications and soft tissue abnormalities (12%).12 Calcifications are more readily detected on mammography than masses or architectural distortion, and it is likely because of this radiographic feature that mammography is more sensitive for detecting DCIS than for detecting invasive breast cancer.2 Calcifications appear as granular or linear deposits that represent intraluminal cellular debris, cellular necrosis, or secretions in the duct epithelium.13 Different forms and patterns of calcifications have been linked with disease biology and prognosis; fine linear or fine linear-branching calcifications seen in a grouped or segmental distribution often represent the DCIS comedo subtype, which has been associated with a higher risk of local recurrence (Fig. 73-1).13–15
FIGURE 73-1:
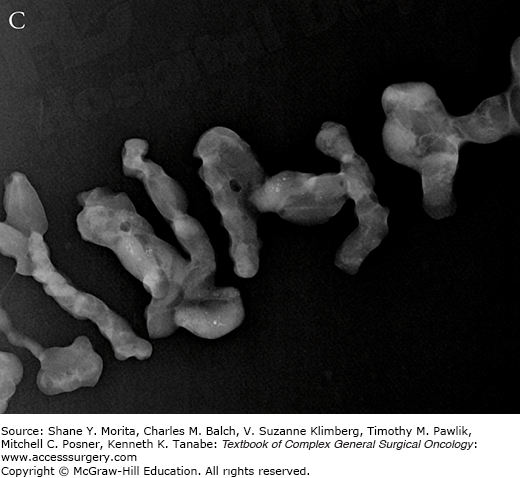
Mammographic features of (A) low-grade DCIS and (B) high-grade DCIS. Note that low-grade lesions are characterized by amorphous loosely grouped calcifications whereas higher grade lesions are more often associated with pleomorphic clusters of calcifications. Specimen radiography is performed after diagnostic core biopsy to confirm adequate sampling of targeted lesion (C).

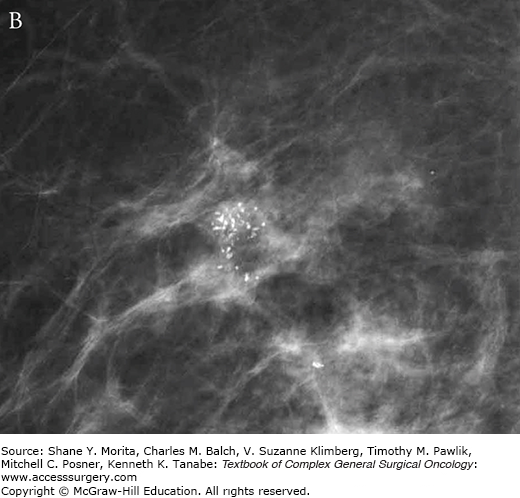
While mammography remains the gold standard for diagnosis and remains the most frequently used tool for the evaluation of patients with DCIS, about 10% of DCIS is mammographically occult.16,17 Furthermore, the inability of mammogram to reliably assess extent of disease, as well as decreased mammographic sensitivity and increased prevalence of cancer in denser breasts, has prompted interest in the investigation of supplemental screening with ultrasound (US) and magnetic resonance imaging (MRI).18 For invasive cancers, US is a valuable adjunct to mammography and clinical examination and has been shown to change surgical management in approximately 10% of patients.18 However, the value of US for DCIS has not been as clear. Studies in women with DCIS who underwent both US and mammogram report 47% to 74% sensitivity of US for DCIS, but this is likely to be an overestimate as radiologists were not blinded to mammography results.19 Overall, multiple studies have reported on the inability of US to accurately characterize the extent of DCIS lesions.18,20
Breast MRI is a highly sensitive imaging modality that is increasingly used in the presurgical evaluation of patients with both invasive cancer and DCIS. The primary objectives of breast MRI are to detect ipsilateral multicentric disease, to evaluate the extent of the known cancer, and to examine the contralateral breast.21 Early studies evaluating MRI for detection of DCIS used noncontrast T2– or T1-weighted images that were not optimal for identifying DCIS.22 However, the use of contrast-enhanced 3D T1-weighted images and refinement of specific diagnostic criteria for DCIS over the past decade has improved the performance of breast MRI in this setting. Although most often presenting as nonmass-like enhancement on MRI, DCIS can also manifest as a mass (14% to 46%) or as a focus (1% to 12%), which is defined as a small area of enhancement that cannot be otherwise classified.14,23,24
A large trial evaluating 7319 German women of average and increased risk with both mammography and MRI revealed that MRI demonstrated greater sensitivity than mammography for all grades of DCIS,25 although the sensitivity of MRI for detection of DCIS was higher for high-grade and intermediate-grade DCIS, compared with low-grade DCIS (98%, 91%, and 80%, respectively). Given the potential for MRI to detect multicentric and contralateral disease, there is concern that widespread adoption of preoperative MRI will lead to an increased number of biopsies, wider excisions, and increasing rates of mastectomy and/or treatment of the contralateral breast. Although there are only a few studies evaluating the effects of preoperative MRI on preoperative management of DCIS, there is an association of MRI with higher mastectomy rates without a significant improvement in re-excision or local recurrence.26–28
Stereotactic vacuum-assisted core needle biopsy (VACNB) has become the preferred method for characterization of suspicious microcalcifications detected on mammography. If a diagnostic MRI of the breast identifies an abnormal area of enhancement, efforts should be made to reidentify the lesion on conventional modalities such as mammography or US.29 VACNB provides architecture for histologic characterization, and distinguishes invasive from in situ disease. Upstaging of atypical or malignant lesions can occur and reach 10% to 50% for ADH, and 4% to 30% for DCIS.30–32 A clip should always be placed at the end of the procedure to allow for mammographic marking if subsequent surgical intervention is indicated. Representative microcalcifications should always be confirmed in the core specimens by specimen radiography.33
Most breast specimens with DCIS do not show an identifiable gross abnormality. However, those DCIS presenting as a palpable or imaging detectable lesion may demonstrate a firm mass. Small round foci of yellow necrosis may be appreciated, corresponding to DCIS ducts displaying comedo necrosis. Histopathologically, DCIS is defined by the World Health Organization (WHO) as a neoplastic intraductal lesion characterized by increased epithelial proliferation, subtle to marked cellular atypia and an inherent but not necessarily obligate tendency for progression to invasive breast cancer.34 In pure DCIS, the intraductal epithelial cells are separated from the breast stroma by an intact layer of basement membrane and myoepithelial cells. DCIS has historically been classified according to histomorphologic appearance. When consensus guidelines were initially drafted for DCIS, architectural features were divided into two main subtypes: comedo- and noncomedo-DCIS based on the presence of cellular necrosis. Noncomedo-DCIS was further subdivided into cribriform, papillary, micropapillary, and solid histologic types, and was generally thought to represent a more indolent group of lesions. Further investigation into biologic markers confirmed that noncomedo subtypes are composed of cells with low-grade cytology, are frequently ER positive and HER2/neu negative, have low proliferation rates, and do not commonly demonstrate angiogenesis or foci of microinvasion. These features directly contrast with the comedo subtype, which is characterized by high nuclear grade cells, central necrosis, and pleomorphic calcifications.
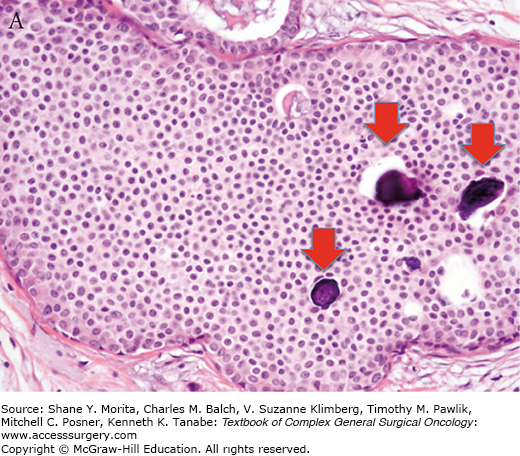
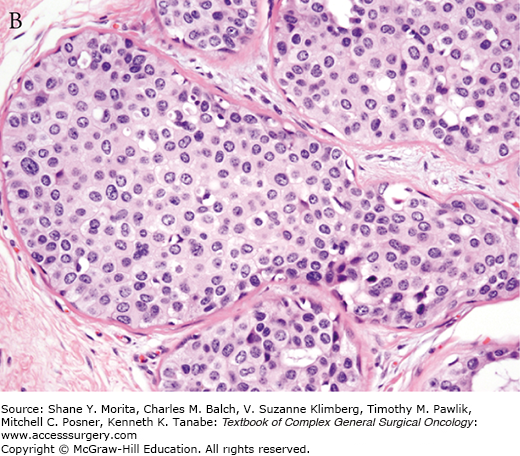
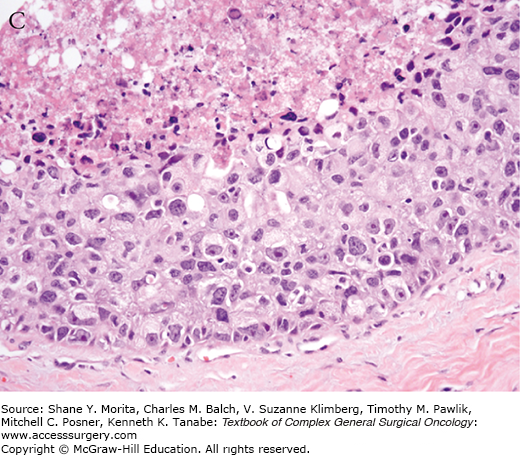
Increasing pathologic experience with DCIS, inconsistencies in classification, and emerging genetic and biologic data eventually led to the current classification schemes using cytonuclear grade alone or in combination with necrosis and/or cell polarization.35,36 Multiple studies evaluating prognostic factors for outcomes of local recurrence and time to recurrence have confirmed nuclear grade as a significant factor.36–39 In 2009, the College of American Pathologists and the American Society for Clinical Oncology established guidelines for pathology reporting of DCIS, requiring defining DCIS lesions as low, intermediate, or high grade, with nuclear grade determined using six morphologic features: pleomorphism, size, chromatin, nucleoli, mitoses, and orientation (Fig. 73-2).40
FIGURE 73-2:
College of Pathology and American Society of Clinical Oncology (CAP/ASCO) criteria for grading of DCIS. Nuclear grade is determined using six morphologic features, including pleomorphism, size, chromatin, nucleoli, mitoses, and orientation. A. Low-grade DCIS. B. Intermediate-grade DCIS. C. High-grade DCIS. Magnification 400×. Arrows indicate foci of microcalcifications.
Another important pathologic criterion is the size or extent of the lesion, which is measured as the greatest distance between the most distant peripherally located DCIS foci. DCIS size is more difficult to assess than that of invasive cancer, due to skip lesions, multifocality, and lack of contiguity of foci compared to invasive cancer. Larger DCIS size has been associated with both increased likelihood of both an invasive component as well as a positive sentinel lymph node. Moreover, time to recurrence is significantly shorter for patients with larger tumors.41 Despite the relationship between size and outcome, DCIS is considered stage 0 or Tis for all lesions without an invasive component. DCIS can extend from the lactiferous ducts into the contiguous nipple skin, a condition also known as Paget’s disease of the nipple. The majority of DCIS cases with Paget disease are HER2-positive. Paget’s disease of the nipple without an invasive carcinoma in the underlying breast parenchyma is staged as Tis.
The presence of microinvasive disease is directly correlated to size and extent of disease, and an important factor in management of DCIS. Microinvasion is defined as the extension of cancer cells beyond the basement membrane with no focus larger than 0.1 cm in diameter and is found in up to a quarter of patients with DCIS, most commonly in larger and higher grade DCIS. However, in long-term follow-up, there appears to be no difference in the recurrence rate or 5-year actuarial survival between those with microinvasion versus those with pure DCIS.42 Multiple studies have looked for an association between microinvasion and axillary node metastases, and the results have ranged from 0% to 38% increased risk.43–46
Important challenges remain in the pathologic assessment of DCIS, particularly in reporting size and margin status. In addition, there are known disagreements between pathologists in how best to distinguish some DCIS from other epithelial lesions such as atypical ductal hyperplasias. However, substantial work has resulted in consensus for pathologic assessment for DCIS including synoptic data elements that have facilitated both research and treatment for this disease.
Most DCIS in the 1960s and 1970s were categorized with invasive breast cancer and were thus treated accordingly with mastectomy and axillary node dissection. However, as DCIS was more frequently diagnosed throughout the 1980s and 1990s, growing data supported that DCIS carried a distinct and different prognosis from invasive cancer, warranting a different surgical approach to its treatment. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 study was designed to assess surgical treatment of invasive breast cancer, but on retrospective review 76 of the 2072 cases were found to be pure DCIS.47 After evaluating this cohort and finding a 43% recurrence rate with lumpectomy alone and 7% recurrence after lumpectomy plus radiation, investigators concluded that the latter was an appropriate approach for DCIS management.48 No other randomized trials have compared mastectomy to BCT plus radiation for DCIS, although observational studies have confirmed that local recurrence rates are lowest after mastectomy.47,48 However, no studies have shown a survival advantage of mastectomy over BCT.3,49,50
According to the most recent SEER data, approximately two-thirds of women diagnosed with DCIS are treated with breast conservation, over half of whom are treated with adjuvant radiation. The goal of breast conservation includes removal of all malignant calcifications and ability to achieve negative margins prior to initiating radiation therapy. As a mostly nonpalpable lesion, radiographic localization with wire, radioactive seed, or hydromark clip is usually required, and confirmation of retrieval of the targeted lesion on a specimen radiograph is essential to a successful outcome.
There have been four randomized clinical trials that have assessed lumpectomy alone compared to lumpectomy plus radiation for DCIS (Table 73-1).51 All four trials demonstrated that the addition of adjuvant radiation after lumpectomy reduced the 10-year absolute risk of an ipsilateral breast event significantly, with a relative long-term risk reduction of over 50% (from 24.6% to 12.2%). The benefit of radiation was seen in all subgroups regardless of age at diagnosis, extent of breast-conserving surgery, use of tamoxifen, method of DCIS detection, margin status, focality, grade, comedonecrosis, architecture, or tumor size. A recent NSABP study showed that women with DCIS who recur with invasive cancer have a twofold greater mortality risk relative to those without invasive recurrence.52 Although the overall risk of local recurrence is low with breast conservation, this study maintained that suboptimal locoregional treatment could impact outcomes. Patients should be informed of the higher risk of local recurrence with breast conservation compared to mastectomy; however the randomized trials have shown that overall prognosis is excellent with breast conservation for DCIS, and adjuvant radiation is recommended for most women who are candidates for BCT.
Randomized Clinical Trials of Lumpectomy versus Lumpectomy and Radiation for DCIS: NSABP B-17,92 EORTC,93 UK/ANZ,94 and the Swedish Breast Cancer Group95
| Study | Year of Study Closure | Median Follow-up (Year) | % Positive or Unknown Margins | Ipsilateral Breast Events/Women | |||
|---|---|---|---|---|---|---|---|
| (n) BCS + RT | % IBTR | (n) BCS Alone | % IBTR | ||||
| NSABP B-17 | 1990 | 16.5 | 13% | 78/400 | 19.5 | 139/398 | 34.9 |
| EORTC 10853 | 1996 | 10.4 | 16% | 64/462 | 13.9 | 118/456 | 25.9 |
| SweDCIS | 1999 | 8.4 | 11% | 59/511 | 11.5 | 131/500 | 26.2 |
| UK/ANZ DCIS | 1998 | 4.8 | … | 28/505 | 5.5 | 67/497 | 13.5 |
| TOTAL | 229/1878 | 12.2 | 455/1851 | 24.6 | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






