Fig. 1
Annual overall number of living donor liver transplants in the United States
3 Comparison of the Results Between LDLT and DDLT
The use of adult LDLT eliminates the prolonged waiting time and decrease the waitlist mortality in patients with HCC in Asia. However, questions are raised by some regarding the higher recurrence rate of HCC after LDLT compared to DDLT, even in patients meeting the Milan criteria, and the risk and voluntarism of living donors [10, 24]. Possible explanations for the higher rate of HCC recurrence among patients who have undergone LDLT relate to the loss of the selection effect of waiting patients with more aggressive tumors by putting them on a fast tract to transplant; a less optimal cancer operation brought about by greater manipulation of the recipient liver prior to transplant to obtain greater length of hepatic artery, portal vein, and bile duct, as well as preservation of the native vena cava; and the angiogenesis-associated phenomenon attributed to the rapid regeneration of a small-for-size liver graft. However, application of the Milan and UCSF criteria to LDLT has resulted in patient survival outcomes very similar to those following DDLT, as shown in high-volume multicenter cohorts from Japan and Korea [25, 26]. Moreover, the prognostic powers of the Milan and UCSF criteria were reported to be the same in both DDLT and LDLT [26].
The multicenter study from Korea comprising 90 % of total LTs from August 1992 to December 2002 showed outcomes of 312 HCC patients diagnosed on the explanted liver. The number of LDLT and DDLT was 237 patients (76 %) and 75 patients (24 %), respectively, and the Milan criteria were met in approximately 70 % of patients in both groups. The 2-year recurrence-free survival rate, however, was not different between LDLT and DDLT after the exclusion of perioperative mortality and incidental HCC. The patients who met or exceeded the Milan criteria showed 3-year survival rates of 89.9 % and 66.4 % after DDLT, and 91.4 % and 62.6 % after LDLT. The results with DDLT and LDLT in regard to HCC recurrence in recipients within the Milan criteria were comparatively similar not only to the original results of DDLT [3] but also to the Japanese results of LDLT [25].
Although Adult-to-Adult LDLT (A2ALL) data reported by Kulik et al. [8] showed higher recurrence rate, it was a limited cohort of patients undergoing transplantation for HCC at Northwestern University Medical Center and the recipient was fast-tracked by performing LDLT, especially in the pre-MELD era in which patients with HCC were disadvantaged by the allocation algorithm [8]. In the West, LDLT is often pursued for patients who do not meet the stringent Milan criteria for MELD exception points who have tumors with an inherently worse prognosis. In addition, updated analysis of the A2ALL cohorts presented at the October 2010 American Association for the Study of Liver Disease meeting which re-analyzed the A2ALL data and concluded that “differences in tumor characteristics and management of HCC in patients who received LDLT likely accounted for the higher HCC recurrence rates observed in their LDLT group”.
4 Proposed Criteria for Use of LDLT for HCC
The acceptable long-term survival rate in DDLT for HCC may need to be at least 70 %, because deceased donor organ supply is too short for the patient’s number on the waiting list and there are many other candidates without HCC for whom DDLT can offer long-term survival probability in excess of 70 %. The Milan and other mild extension criteria, like the UCSF, have been validated satisfying former acceptable outcomes by many DDLT centers. To date, the Milan criteria is regarded as the gold standard for selection of HCC patients for DDLT.
In the LDLT scenario, however, a live donor graft is a dedicated gift that is directed exclusively to a particular beloved person who has a life-threatening disease and there is no need for an objective allocation system based on a prioritization scheme. LDLT is virtually the only option for patients with HCC in Asian countries, where deceased donors are limited [27], and for patients exceeding the Milan criteria in Western countries [28]. The decision for LDLT for HCC, therefore, is exclusively based on the balance between donor risks and recipient benefits, and expansion beyond the standard criteria (Milan and UCSF criteria) to more advanced tumor staging in patient selection has been proposed in many Asian LDLT centers (Table 1). Those criteria are based on the independent predictors for outcome derived from the analysis of the pretransplant factors and explant pathology.
Table 1
Eligibility criteria of living donor LT for HCC
Institution | Selection criteria | Biologic markers |
|---|---|---|
Asan, Korea | All tumor ≤5 cm and number ≤6 | Very high risk of recurrence meets more than three among four conditions. ≥5 cm, AFP ≥1,000 ng/mL, PET+, poor histologic grade on pre-LT biopsy |
Hong Kong, China | No diffuse tumor or gross vascular invasion | |
Multicenter, Japan | Single ≤5 cm, or number ≤3 and size ≤3 cm | And AFP ≤400 ng/mL and PIVKA II ≤100 mAU/mL |
Kyoto, Japan | All tumor ≤5 cm and number ≤0 | And PIVKA II ≤400 mAU/mL |
Kyushu, Japan | All tumors <5cm | Or PIVKA II <300 mAU/mL |
Samsung, Korea | All tumor ≤5 cm and any number | And PIVKA II ≤400 mAU/mL |
Seoul University, Korea | All tumor ≤5 cm and number ≤10 | And PIVKA II ≤400 mAU/mL |
Tokyo, Japan | All tumor ≤5 cm and number ≤5 |
The Asan group has set the extended criteria that include largest tumor diameter 5 cm, tumor number up to six, and no gross vascular invasion. As of December 2004, a total of 221 HCC patients had undergone LDLT at Asan Medical Center. The independent risk factors for HCC recurrence were the largest tumor diameter more than 5 cm, tumor number more than six, and gross vascular invasion. A comparison of patient survival rates based on the Milan, UCSF, and Asan criteria showed similar prognostic power, but Asan criteria demonstrated the highest discriminatory power that overall 5-year patient survival rates were 76.3 % and only 18.9 % within and beyond the Asan criteria, respectively [29]. In addition, the incorporation of tumor size more than 5 cm, serum AFP more than 1000 ng/ml, positive positron emission tomography using 18F-fluorodeoxyglucose (18F-FDG-PET), and poor histologic grade by pretransplant tumor biopsy into a scoring system as selection criteria for excluding HCC patients for LDLT could help to identify the patient group at very high risk of HCC recurrence. Such parameters of the biologic behavior of tumors, therefore, will help in decision making about the inclusion and exclusion of LDLT candidates with HCC beyond the current expanded selection criteria [15]. Kyoto group reported the results of LDLT in 125 patients with HCC, showing that patients with up to 10 tumors <5 cm in diameter and with a protein induced by vitamin K antagonist (PIVKA) II value <400 mAU/ml enjoyed a 5-year survival of 86.7 %, whereas those exceeding these criteria had a 5-year survival of 34.4 % [30]. The Kyushu group reported a series of 90 patients who underwent LDLT for HCC, in whom the number of tumors did not correlate with the prognosis, but patients with a tumor diameter (>5 cm) or PIVKA II (≥300 mAU/mL) had a significantly poor prognosis [31]. The Tokyo group applied the so-called “5–5 rule” by limiting LDLT to patients with up to five nodules with a maximum diameter of 5 cm. The recurrence free 3-year survival of patients who met the criteria was 94 % versus 50 % in those patients exceeding the 5–5 rule [32]. The Japanese 49 multicenter study including 653 patients with HCC who received LDLT proposed new selection criteria using three variables: within the Milan; low serum AFP (≤200 ng/mL); and low PIVKA II (≤100 mAU/mL). The 5-year disease-free survival rate among the 124 patients who were beyond Milan but satisfied low AFP and PIVKA II level was 84.35 % [33]. The Hong Kong group has selectively included advanced HCC patients since 2006 as extended indication criteria for LDLT when HCC patients did not have following exclusion criteria: (1) gross vascular invasion, (2) evidence of distant metastasis, (3) evidence of diffuse HCC [34].
Although application of the UCSF criteria increases inclusion rates by 5–10 % compared to the Milan criteria, these proposed extended criteria in LDLT included more HCC patients having prolonged survival after LT. The higher inclusion rates compared to the application of the Milan criteria are achieved by criteria which include biologic tumor marker, either AFP or PIVKA II or positron emission tomography. Inclusion of tumor markers in addition to parameters of tumor morphology might be the key to establishing the best criteria for LT for HCC.
5 Pretransplant Treatment
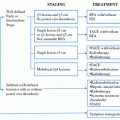
Hepatectomy and locoregional treatments such as transarterial chemoembolization (TACE), percutaneous ethanol injection(PEI), and radiofrequency ablation (RFA) have been used in patients with HCC according to the tumor characteristics and the status of the remaining liver function. A considerable proportion (75 %) of LDLT recipients with HCC underwent those treatments before LT, and LDLT as a primary treatment for HCC patients is not common except when LDLT is performed in patients with severely decompensated liver function because finding an available living donor is not easy [29].
Pretransplant treatment for HCC in LT is performed as (1) a bridging therapy for preventing dropouts from waiting list, and for improving the posttransplant survival by selecting out patients whose disease will recur or by stopping the progression of the tumor before extrahepatic spread has occurred, or (2) downstaging the tumor to meet currently available criteria such as Milan, UCSF, or Asan, etc. In case of LDLT, pretreatment for HCC has been performed not to dropout but to improve survival or downstage the tumor.
Bridging therapy refers to the treatments implemented in patients who already qualify for transplantation according to accepted selection criteria until they receive a graft [35]. Locoregional treatment such as TACE, RFA, and PEI is used to provide complete tumor necrosis in an attempt to halt tumor progression and gives a modest survival benefit [36, 37]. As a result most units offer dual as well as single modality ablative treatments, in an attempt to reduce tumor progression and recurrence rates. Hepatic resection has also been used as a bridge to transplantation [38]. Unlike locoregional treatment which has been shown in studies to achieve only partial tumor necrosis, resection should achieve the best tumor control. Resection also allows a thorough intra-operative assessment of liver status and tumor burden, and is associated with increased risk and, as stated earlier and should only be attempted in well-compensated cirrhotic patients. Under the scarcity of cadaveric organ donation in Asia, most of LT centers cannot adopt the strategy of bridging therapy but have to use those multi-modality treatments as an alternative to curative treatment or as a downstaging therapy before LDLT.
Of the 653 recipients in the Japanese multicenter study, 466(71.4 %) received various adjuvant treatments, alone or in combination, before LDLT [33]. Neither pretransplant treatments nor the type of modalities showed any influence on the patient survival and recurrence rate compared with those of the recipients who received no treatment. An intriguing study was reported by Takada et al. [12]. When 93 patients with HCC who had undergone LDLT were divided into three groups based on the number of pretransplant therapy, it reflected differences in the median time elapsed from the diagnosis of HCC to LDLT. The patients who received only one or two previous treatment had the best 4-year survival (80 %) and the lowest recurrence rate (9 %). It seems that one or two sessions of pretransplant treatment is associated with improved morphologic characteristics and reduced biologic aggressiveness of HCC.
The debate regarding the choice of primary transplantation versus primary hepatectomy followed by salvage transplantation is still ongoing due to the conflicting results from two French groups [39, 40]. In Asia, Hwang et al. [41] have shown that overall survival rate after salvage LDLT was similar to that after primary LDLT, particularly when the extent of recurrent tumor was within the Milan criteria. Technical feasibility, however, should be taken into account for salvage LDLT due to adhesion and anatomical distortion in the abdomen, although these features could be overcome by technical thoroughness. Laparoscopic liver resection seems to have a benefit to facilitate late LT, although its eligibility is limited [42]. There is still no consensus on the eligibility criteria for salvage LDLT regarding tumor extent and interval after prior resection, but it has been suggested that the same criteria may be shared for primary and salvage LDLTs [41].
Downstaging treatments has been performed for patients initially beyond Milan criteria and in order to meet Milan criteria for DDLT mostly in Western countries [43–47]. Yao et al. reported excellent LT outcomes following downstaging of HCC that was even better than outcomes previously reported for patients who met the Milan criteria [39]. There have been no prospective results of LDLT following downstaging of HCC. Moon et al. from Asan Medical Center reported a retrospective study about the survival differences between Artificial Milan group after downstaging (22 patients) and De novo Milan group (65 patients) in LDLT for HCC [48]. Five-year cumulative survival rates were not different between them (83.9 % versus 93.9 %), but 5-year disease-free survival rates were significantly different (71.1 % versus 96.5 %). As a result, more strict follow-up is necessary in Artificial Milan group.
6 Postoperative Surveillance and Management for HCC Recurrence After LDLT
Reported risk factors for HCC recurrence include tumor size and number, bilobar spread of tumor, elevated serum AFP and PIVKA II levels, poorly differentiated HCC, positive lymph nodes, and vascular invasion [30, 49–54]. Most centers apply strict indication criteria such as the Milan or UCSF, etc. for LT of HCC based on the preoperatively measurable risk factors [26, 30, 53, 54]. HCC recurs in 10–20 % of transplant recipients, however, despite careful patient selection [3, 25, 55, 56]. Once recurrence occurs, survival time is usually less than 1 year [55, 56]. Early detection and aggressive treatment for posttransplant HCC recurrence, however, can result in prolongation of patient survival in a considerable proportion of patients [55–57]. Tumor markers such as AFP and PIVKA II and multimodal imaging studies including dynamic computed tomography (CT) scan of abdomen, enhanced chest CT scan, head CT, 18F-FDG-PET, and bone scintigraphy are useful to detect HCC recurrence.
Yamashiki N et al. reported that AFP levels decreased immediately post transplantation. In all cases without HCC recurrence, AFP levels decreased to lower than 20 ng/mL within 2 months post transplantation. When cutoff level was 10 ng/mL compared to 20 ng/mL, the sensitivity is better and the specificity is almost same. Unlike the AFP, a transient increase in PIVKA II levels without evidence of recurrence was often observed postoperatively. The causes of a PIVKA II increase to over 40 mAU/mL included the following: biliary obstruction, oral administration of warfarin, and unknown. When the cutoff values were 10 ng/mL for AFP and 40 mAU/mL for PIVKA II, the sensitivity of the combination increased to 100 % and specificity was 91 %. The optimal frequency, however, is not clear. Because most of the recurrent cases were diagnosed within 2 years, monthly measurement would be useful for the first 1–2 years after transplantation [58]. As each HCC patient is at a different degree of HCC recurrence after transplantation, the Asan group perform follow-up studies including AFP, PIVKA II, liver dynamic CT scanning, and chest X-ray as a primary evaluation and the frequency is decided by the risk level. Advanced HCC patients beyond Asan criteria are at the high risk of recurrence during the first year, and such patients are more strictly monitored, especially during that period. 18F-FDG-PET and chest CT scan were performed immediately in patient suspected of HCC recurrence by analysis of tumor marker or upon imaging. In the meanwhile, 87 patients meeting super-selection category defined by small untreated HCC patients with ≤2.0 cm in size, ≤2 nodules, and AFP ≤200 ng/mL had 1.3 % 5-year recurrence and 92.1 % 5-year survival rates. As a result, tumor markers including AFP and PIVKA II, and liver dynamic CT can be followed less frequently for cost-effective post-transplantation surveillance for HCC recurrence [59].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree