© Springer Science+Business Media Singapore 2017
Hiroki Yamaue (ed.)Innovation of Diagnosis and Treatment for Pancreatic Cancerhttps://doi.org/10.1007/978-981-10-2486-3_1212. Distal Pancreatectomy for Pancreatic Carcinoma
(1)
Department of Surgery, Nara Medical University, 840 Shijo-cho, Kashihara Nara, 634-8522, Japan
Keywords
Distal pancreatectomyPancreatic adenocarcinomaRAMPSLaparoscopic pancreatectomyRobotic pancreatectomy12.1 Introduction
Distal pancreatectomy (DP) has been widely used as the standard treatment for carcinoma of the pancreatic body and tail. However, due to the position of the body and tail of the pancreas within the anatomy, tumors in this area may develop before the appearance of clinical symptoms such as pain, weight loss, diarrhea, and appetite loss. Furthermore, once these symptoms appear the cancer is usually at an advanced stage, often with distant metastases including the liver and peritoneal metastases. Therefore, most patients with carcinoma of the pancreatic body or tail are often not operable.
The first left-sided pancreatectomies, namely, DP, were performed in the late 19th century in Europe and the United States [1]. DP entails the removal of the portion of the pancreas extending to the left of the midline and not including the duodenum and distal bile duct. The pancreas is usually divided into the left of the superior mesenteric vein or portal vein trunk, with the exact line of transection depending on the location of the tumor. Regional lymphadenectomy is usually performed concomitantly. Spleen-preserving DP is generally contraindicated for carcinoma of the distal pancreas. DP for pancreatic carcinoma aims to achieve negative surgical margin.
12.2 Postoperative Complication and Pancreatic Fistula After Distal Pancreatectomy
There are distinct differences in the postoperative course of DP, compared to pancreatoduodenectomy (PD). In a cohort of 2322 DP patients from the American College of Surgeons National Surgical Quality Improvement Program, 28.1% experienced postoperative complications, and 30-day mortality was 1.2% [2]. In a cohort of 11,559 patients reported by the Pancreatic Surgery Mortality Study Group, 218 (1.9%) mortalities followed pancreatectomy. Eighteen (8.3%) of all mortalities followed DP. Among the cause of death, operation-related complications were fewer (5.5% vs. 28.3%), and postoperative pancreatic fistulas (PF) was comparable (11% vs. 15.2%) to PD. In contrast, disease progression (11% vs. 4.4%), pulmonary embolism (11% vs. 0.5%), and medical conditions (11% vs. 1.6%) contributed more frequently [3]. In a cohort of 2360 patients of the Pancreatectomy Readmission Assessment Group Study, 464 (19.7%) patients, including 317 PD and 147 other pancreatectomies, readmitted within 90 days. Many medical conditions and postoperative variables predicted readmission; however, there were no differences in intraoperative variables between the readmission group and no readmission group [4].
Among a number of postoperative complications including intra-abdominal abscess, wound infection, sepsis, malabsorption, and hemorrhage, PF is still the main cause of postoperative morbidity after pancreatectomy. A recent study assessed the clinical impact of PF using a complication severity grading system, and then compared PF after PD and DP [5]. In 2370 pancreatic resections, PF (34.5 vs. 27.2%, P < 0.001), as well as clinically significant PF (14.7 vs. 11.4, P = 0.019) was more frequent in DP than PD. Furthermore, in DP, PF consisted 31.2% of the overall complication burden, compared to 17.5% in PD. Shown from the data above, although operation-related mortality was less experienced in DP, the postoperative course was characterized by the high incidence and burden of PF.
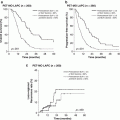
Many large-scale, multi-institutional studies show the negative effect of postoperative complications following pancreatectomy to the long-term survival [6, 7]. In 761 distal pancreatectomies for pancreatic adenocarcinoma with a median survival of 17 months by the Dutch Pancreatic Cancer Group [6], the 33% of patients who developed major postoperative complications had a significant risk of worse survival (HR 1.67, P = 0.02). In 1397 pancreatectomies for pancreatic cancer by the Multicenter Study Group of Pancreatobiliary Surgery [7], there were more distal lesions in Grade B and C compared to Grade A (54.4% vs. 43% vs. 27.6%, P < 0.001), and PF Grade C, but not Grade B, was an independent risk factor for overall survival (HR 1.59, P = 0.035). Although it is generally thought that reducing morbidity and prompting early postoperative recovery is important in the surgical management of malignant disease, early induction of adjuvant therapy for pancreatic cancer may not be associated with long-term survival [8]. Therefore, the main objective of early recovery from surgery would not necessarily be the early initiation of adjuvant therapy. However, severe complication could hinder the completion of the planned postoperative adjuvant therapy, as well as worsen the overall survival [7]. Kawai et al. report the rate of postoperative therapy was 38% in PF C, in comparison to 81.5% in PF A, and 84.6% in PF B. The mechanisms that cause poor prognosis after severe complication are not fully elucidated. However, it is assumed that an immunosuppressive effect by inflammatory cytokines has a role in tumor progression [9]. It may also be speculated that physical deterioration leads to intolerance of adjuvant therapy administration [7]. Nonetheless, the sustaining effect from postoperative complications may interfere with the sequencing of multimodal therapy, which is vital for the long-term survival of pancreatic cancer. Taken together, especially in DP for pancreatic cancer, all efforts to pursue lower morbidity, namely, PF, may lead to the improvement of the oncological outcomes as well. This highlights the importance of high surgical technique, as well as collective experience of the attending team, in the surgical management of pancreatic cancer.
Hospital volume, as well as surgeon volume has been reported to be associated with lower morbidity and mortality after pancreatectomy in many large studies with heterogeneous backgrounds [10–12]. Volume–outcome relationships are well investigated in PD. On the other hand, the direct impact of volume to postoperative complications in DP is largely unknown. Therefore, it is still unclear whether higher surgeon volume or superior surgical technique will contribute to decrease morbidity, as well as PF, in DP. Regarding the impact of hospital volume to prognosis after pancreatectomy for malignant disease, Derogar et al. showed the tendency of longer prognosis in high volume centers [10]. Moreover, they show the significant difference of prognosis between educational and noneducational institutes. The advantage of centralizing patients is, high-volume centers may be able to provide more robust, diversified therapy including multimodal therapy, greater lymph node harvesting, higher margin-negative resections, and detailed histological evaluation. Furthermore, educational institutes may have advantages such as better adherence to clinical guidelines, improved patient selection, multidisciplinary team management, availability to novel technologies, and concentration of high-volume surgeons [10]. Many high-volume centers report the improvement of short-term, as well as long-term outcomes over the period of time [13].
Taken from above, for the improvement of outcome for pancreatic cancer, equally important to the role of surgeons, is the total quality of high experienced institutions. Furthermore, treatment strategies that are unique to pancreatic cancer may effect postoperative complication after pancreatectomy. Multimodal therapy is becoming recognized as an important treatment strategy for pancreatic cancer. Although it is presumably regarded that preoperative therapy is associated with increased morbidity, in fact, PF as well as morbidity, has been reported to be fewer in neoadjuvant radiotherapy patients [14, 15]. Preoperative radiation has been reported to be associated with significantly lower incidence of PF after DP, possibly due to the induction of atrophy and the distortion of lobular structure with acinar cell dropout [16].
12.3 Management of Pancreatic Stump: Suture, Instrumental, Anastomosis
Surgical techniques and methods are the primary and most important factors that influence postoperative morbidity. In DP, leakage from the stump of the remnant pancreas is the direct cause of PF and leads to severe complications such as intra-abdominal abscess and postoperative hemorrhage. Therefore, it is reasonable that pancreatic surgeons have always devoted much effort to evaluating the optimal pancreatic stump closure method. However, in spite of much inspection, the optimal closure method for DP is not yet determined.
The conventional method for pancreatic parenchyma dissection and remnant pancreatic stump closure is scalpel dissection, followed by ligation of the main pancreatic duct, and then hand-sewn closure. Surgeons have since applied numerous developed novel surgical devices and technologies, in attempt to decrease PF. Surgical techniques may be categorized as (1) stump closure method, (2) stump reinforcement method, (3) parenchyma dissection method, and (4) medication administration. Many unique explorations have been made, but the many of these practices are single institute, observational studies. Several randomized control trials could be identified in the literature. Two evaluated stump closure [17, 18], six evaluated stump reinforcement [19–24], two evaluated somatostatin analogues [25, 26], and one regarded parenchyma dissection method [27]. Furthermore, one prospective controlled clinical trial sought to determine whether prophylactic transpapillary pancreatic duct stenting reduces PF after DP [28]. Fifty-eight patients were randomized to either DP or DP plus stent. Clinically significant PF (Grade B/C) occurred in 6 DP and 11 DP plus stent patients. The results indicated that prophylactic pancreatic stenting did not reduce PF when performing a standardized resection of the body and tail of the pancreas.
The greatest alternative for the hand-sewn method is closure by stapler. Stapler resection can be a safe, fast method, and moreover, can also be applied to minimally invasive surgery. From the technical point of view, it has become favored and widely used in clinical practice. However, in terms of postoperative complications associated to the device, the rate of PF and mortality did not differ with conventional hand-sewn technique [17]. Increased parenchyma compressing injury in a thick pancreas may be a drawback in the stapling method [29]. Kawai et al. hypothesized that a seromuscular patch of the pancreatic stump concomitant with pancreatic duct anastomosis, namely, pancreaticojejunostomy (PJ), could be effective, compared to the stapler method as control. In their RCT, although the overall PF did not differ between the PJ and stapler groups, in a subgroup analysis of a thick pancreas subset, the PJ group trended to have lower PF [18]. The authors advocated further RCTs with stratification by the pancreas thickness.
Although the optimal stump closure method for lowering morbidity is unresolved, the stapler technique remains to be the mainstream method of choice. Many RCTs have evaluated the efficacy of reinforcing the remnant stump. However, the use of falciform patch [20] (20% vs. 19.6%), fibrin sealant patch [19, 22] (62% vs. 68%, 54.5% vs. 56.6%), or seromuscular patch [23] (8.6% vs. 20%) neither had additive protective effect. One RCT showed the significant reduction of clinically relevant PF by reinforcing the staple line with mesh buttress material [21]. PF B/C was 1.9% with mesh reinforcement versus 20% in the control group. Limitations to the study include, that though the study did not specify any stratification by pancreas thickness or texture hardness, some patients were excluded, on account of inability to safely apply stapler in thick pancreas, and also in proximal lesion.
The only other RCT that showed positive results was the administration of prophylactic pasireotide [25]. This study clearly shows that pasireotide reduces PF associated severe complications in any subset of pancreatectomy, including PD, DP, and dilated or non-dilated duct. Furthermore in DP, PF B/C was 7.9% with pasireotide versus 16.9% in control. Although there were some concerns regarding cost-effectiveness [30], it was useful to reduce any complication, which was 11.2% with pasireotide, and 25% in control. A recent meta-analysis by the Cochrane Upper GI and Pancreatic Disease Group evaluated 19 studies on the efficacy of somatostatin analogue use in pancreatic surgery [31]. As a result, all PF was reduced, but clinically significant PF was not significantly different. Furthermore, although mortality rate was comparative, since total complication rate was lower with somatostatin, it was recommended for routine use in pancreatectomy.
12.4 Standard Distal Pancreatectomy Versus RAMPS Procedure for Pancreatic Carcinoma
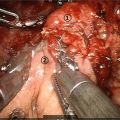
Strasberg et al. have established a surgical procedure of left-sided pancreatectomy for pancreatic carcinoma, radical antegrade modular pancreatosplenectomy (RAMPS) [32]. RAMPS was designed to achieve the two goals of pancreatic resection for pancreatic cancer, namely, negative dissection planes and regional lymph node resection. The main concepts of RAMPS are: (1) complete resection of N1 lymph nodes, (2) emphasis on posterior margin, (3) dissection in a right-to-left manner. The lymph nodes that circulate the pancreas body and tail, as well as their drainage nodes in the celiac and superior mesenteric arteries are regarded as N1 nodes. Posterior dissection planes were designed according to the relationship of the retroperitoneal fascia planes between the pancreas posterior and anterior of the left kidney. Depending on whether or not the tumor extends to or past the posterior pancreatic capsule, the dissection plane border is in the perirenal space at the anterior of the adrenal gland (anterior RAMPS), or behind the adrenal gland and Gerota fascia (posterior RAMPS). Right-to-left dissection enables early control of major veins, as well as simplifies the identification of anatomical landmarks that indicate the posterior dissection plane.
The procedure is summarized as follows: After dividing and closing the pancreas neck, celiac and superior mesenteric nodes are dissected posteriorly. The splenic vein and artery are ligated and divided, then dissection of fat is done further down, visualizing the celiac axis, superior mesenteric artery origin, and left anterior of the aorta, while resecting lymph nodes between them. Dissection is then done towards the left, according to the predetermined plane. In anterior RAMPS, the left adrenal vein and adrenal gland surface is visualized at the inferior border of dissection. In posterior RAMPS, the left adrenal vein is divided at the origin at the left renal vein, dissection is continued down until the left renal artery, and then turned leftwards in the plane behind the adrenal gland. The inferior border is the left renal artery, left renal vein, diaphragm, retroperitoneal muscle layers, and left kidney surface.
The Strasberg group have achieved negative tangential margins in 94%, with an overall R0 rate of 85%, and a mean 20 lymph nodes were resected in 78 patients [33]. Other groups report R0 rate as 77–90.5%, and lymph node dissection count median 14–26, or mean 15–28.4 [34–36]. In some single-institute series [34, 36] that compare RAMPS with standard DP, RAMPS is associated with greater lymph node count [28.4 ± 11.6 vs. 20.7 ± 10.1, P = 0.001, 14 (5–52) vs. 9 (1–36), P = 0.002], as well as higher R0 rate (90.5% vs. 67.5%, P = 0.005, 89.4% vs. 85.1%). Blood loss was also lower in RAMPS [485 ± 63 vs. 682 ± 72, P = 0.04, 325 (50–3400) vs. 400 (50–3300)]. These data indicate that RAMPS is effectively fulfilling the intended concept of design. Furthermore, it is suggested that the improved local control by RAMPS in combination with multimodal therapy may have prognostic impact, and that RAMPS may have an important role in the treatment strategy for pancreatic carcinoma in the distal pancreas. However, to date, the survival benefit of RAMPS is controversial [34, 36]. Further large-scale studies are warranted to evaluate the role of RAMPS.
The Strasberg group also reports 11 patients by laparoscopic-RAMPS [33]. However, in their experience, satisfactory lymph node cleaning in accordance with the RAMPS concept was hard to achieve, resulting in relatively high conversion rate. Furthermore, the Yonsei group also reported the initial experience with laparoscopic and robotic RAMPS [37]. They concluded that minimally invasive RAMPS is not only technically feasible but also oncologically safe in well-selected patients with left-sided pancreatic cancer. However, the evaluation of feasibility and oncological efficacy of minimally invasive RAMPS is still in an exploratory phase.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree