Distal Femoral Resections with Endoprosthetic Replacement
Jeffrey J. Eckardt
Martin M. Malawer
Jacob Bickels
Piya Kiatisevi
BACKGROUND
Ralph C. Marcove (Memorial Sloan Kettering Cancer Center) and Kenneth C. Francis (New York University Medical Center) introduced limb-sparing resection in the early 1970s for the management of malignant bone tumors, initially for osteosarcoma of the distal femur. The introduction of effective chemotherapy agents (doxorubicin [Adriamycin] and methotrexate) at the same time was a major impetus to the development of these procedures. These surgeons hoped that by combining surgery with chemotherapy, either preoperatively or postoperatively (termed adjuvant chemotherapy), limb-sparing surgery would be safe for the patient and would permit a limb-sparing resection.
Distal femoral endoprosthetic reconstruction has undergone an evolution of surgical techniques and manufacturing changes (initially by Howmedica, Inc., Rutherford, NJ), making it one of the most satisfying orthopaedic oncology procedures available today. Forging of components has greatly diminished mechanical failure problems, and modularity has increased the indications for its use. Musclesparing and soft tissue coverage techniques have minimized wound healing problems.
The three major steps in limb-sparing surgery—wide excision with good oncologic margins, reliable reconstruction of the skeletal defect, and adequate muscle transfer and good prosthetic coverage—have formed the basis for reliable and safe limb-sparing resections and reconstruction for both low- and high-grade bone sarcomas. Most clinical experience has been gained in treating osteosarcoma of the bone. The most common site is the distal femur and the proximal tibia. These techniques have subsequently been used for other bony sarcomas and recurrent benign tumors and more recently in the treatment of failed allograft and complicated, multifailed total knee arthroplasties.
The goal is to have an adequate oncologic resection while maintaining enough muscle to permit a painless functional result. The techniques outlined in this chapter are based on the senior authors’ (MM, JJE) 51 years of combined surgical experience, with approximately 440 distal femoral reconstructions since 1979.
ANATOMY
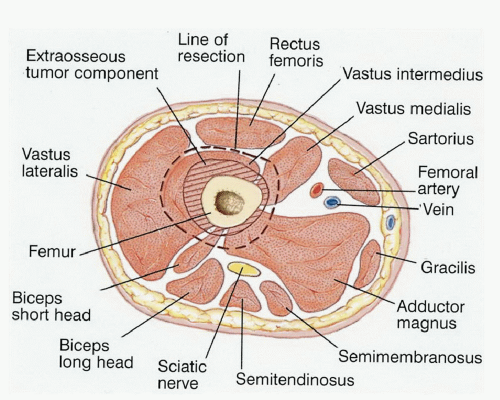
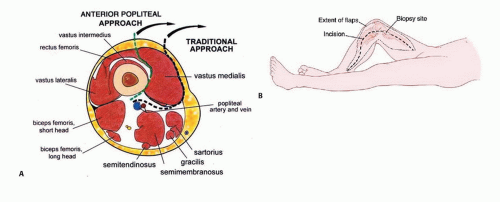
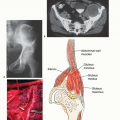
The surgeon must be extremely knowledgeable of not only the bony anatomy and the specific endoprosthesis to be used but also the vascular anatomy, soft tissue structures, and potential local muscle flaps and the many techniques involved in a limb-sparing resection (FIG 1).
Sartorial Canal
The sartorial canal occupies the space between the vastus medialis, sartorius, and adductor magnus muscles in which the superficial femoral artery passes the medial aspect of the thigh (adductor hiatus) and then enters the popliteal space.
In patients with tumors longer than 13 cm, the sartorial canal is often displaced. The vessels within the canal are usually protected by the deep fascia of the vastus medialis and a tough fascia surrounding the vessels. This fascia border is rarely penetrated by tumor.
Knee Joint
The knee joint is rarely directly involved by sarcoma. The main mechanisms of knee joint contamination are inappropriate biopsy, extension of tumor along the intra-articular cruciate ligaments, and pathologic fracture. The knee joint can reliably be evaluated by computed tomography (CT) and magnetic resonance imaging (MRI).
If the physical examination reveals any evidence of effusion, the knee joint should be aspirated and histologic samples obtained. A hemarthrosis usually indicates tumor involvement of the synovium. This is a rare event but not an indication for amputation.
Popliteal Space
The popliteal space contains the popliteal artery and vein and the sciatic nerve. The popliteal vessels enter the popliteal
space from the medial aspect through the adductor hiatus as the vessels exit the sartorial canal. The popliteal vessels are evaluated by CT with contrast, MRI, and plain angiography.
It is rare to have direct vessel involvement by tumor. The vessels may be displaced as the tumor grows posteriorly, but usually, there is a normal border or margin of popliteal fat.
Exploration of the popliteal space is the first step in determining the feasibility of a limb-sparing procedure. The popliteal vessels are dissected out and the geniculate vessels are ligated. If the vessels are free of tumor, resection can usually be performed safely.
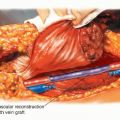
A frozen section of the popliteal fat or adventitia of the popliteal vessels should be obtained intraoperatively. If there is obvious vascular involvement, the vessels can be replaced by vascular graft.
The popliteal vein is usually not repaired because it rarely stays patent after surgery.
Anterior and Posterior Cruciate Ligaments
The cruciate ligaments are occasionally involved by direct tumor extension from the distal femur. This occurs through the bone-tendinous junction of the intercondylar notch of the distal femur. There is no cartilage in this area to act as a barrier to tumor growth.
MRI is occasionally helpful in determining cruciate ligament involvement.
Tumor nodules of the anterior and posterior cruciates occasionally present with a hemarthrosis. The most common finding at resection is tumor nodule involvement of the cruciates. This does not rule out a limb-sparing procedure. The cruciate ligaments as they attach to the proximal tibial plateau can be resected en bloc with the proximal tibial cut. This is a safe procedure that avoids the need for a true extraarticular resection.
INDICATIONS
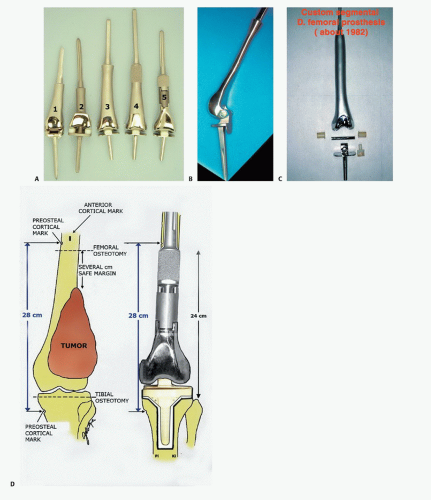
Endoprostheses were initially used solely for reconstruction after resection of malignant bone tumors. Manufacturing time could be as long as 3 months, an interval that permitted induction chemotherapy. Endoprosthetic reconstructions proved to be enduring, and the designs have evolved (FIG 2).5,6
Modularity, which made for immediate availability, permitted the expansion of the indications for distal femoral endoprosthetic reconstruction to some stage 3 giant cell tumors of bone; metastatic disease where conventional intralesional procedures cannot reasonably be done, possibly 10% of metastatic cases; complex supracondylar fractures in elderly osteoporotic patients; failed internal fixation of distal femoral fractures; failed allograft or total knee reconstructions; and as a primary knee replacement system in patients with a severe flexion contracture where bone and ligament resection would lead to instability with conventional knee replacement systems.
PATIENT HISTORY AND PHYSICAL FINDINGS
The average age of patients with high-grade osteosarcomas is 5 to 30 years; the median is 16 to 21 years. Surface osteosarcomas occur in the third decade and are more common in women.
Patients with high-grade osteosarcoma almost always initially complain of pain during the day that is not associated with activity. All patients complain of a dull aching pain and only later of night pain.
Thirty percent to 40% of patients have a history of local trauma. There is no causal relationship of trauma to the tumor except that it brings the patient to medical attention and the physician orders a radiograph, which always shows the tumor. This has been termed traumatic determinism.
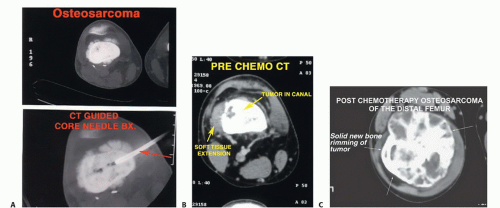
Classic high-grade osteosarcoma presents with pain. Parosteal osteosarcoma (surface osteosarcoma) usually presents with a mass and not pain (FIG 3).
Parosteal osteosarcomas are most common in the posterior aspect of the distal femur. They represent less than 4% of all osteosarcomas. Popliteal fullness is a common finding. Plain radiographs can often distinguish a classic from a parosteal osteosarcoma.
There may be tenderness on examination. The regional lymph nodes are normal. Osteosarcomas spread hematogenously. Infection is rarely a consideration.
Pathologic fracture occurs in less than 1% of osteosarcomas. Fractures usually occur through the purely osteolytic variant (about 25% of all osteosarcomas), which has minimal mineralized tumor matrix.
A soft tissue, extraosseous mass occurs in more than 90% of high-grade osteosarcomas.
An effusion usually indicates tumor involvement of the joint or pathologic fracture.
Distal pulses are usually normal and symmetric. Decreased pulses may represent tumor involvement.
Leg edema may represent popliteal vein occlusion or thrombus.
Enlarged groin lymph nodes may represent lymph node metastasis, but this is rare. Biopsy should be considered.
Popliteal lymph node enlargement is extremely rare (except for Ewing sarcoma or lymphoma).
IMAGING AND OTHER STAGING STUDIES
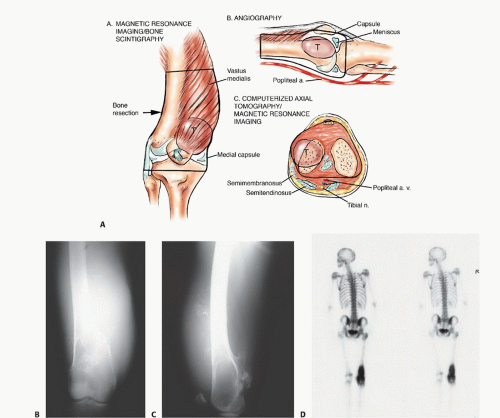
Diagnostic imaging should include plain radiographs, a technetium 99 bone scan, an MRI of the entire femur, and a CT scan of the distal femur (FIG 4), as well as angiography. Three-dimensional CT angiograms have recently replaced routine angiography. Preoperative staging studies focus on the four anatomic structures discussed earlier. This permits the surgical team to determine the type of surgery, placement of the incision, the need for intra- or extra-articular resection, and the biopsy site and technique.
Plain radiographs correlate very well with the extent of the tumor when a Codman triangle is present.
A technetium 99 scan shows the extent of the tumor within the femur as well as the presence of skip metastases. Multicentric disease or metastases to other bones can be determined from this test as well. The early and pool phases of the bone scan demonstrate the vascularity of the tumor and tend to correlate with the chemotherapy effect (ie, tumor necrosis).
A femoral MRI best shows the extraosseous extent of the tumor as well as its proximal and distal extent within the medullary canal. This study is the most accurate in detecting skip metastases.
CT scans are complementary to MRI scans and can show the quality of the bone stock at the intended level of resection.
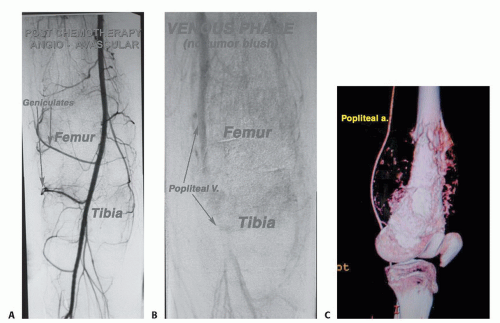
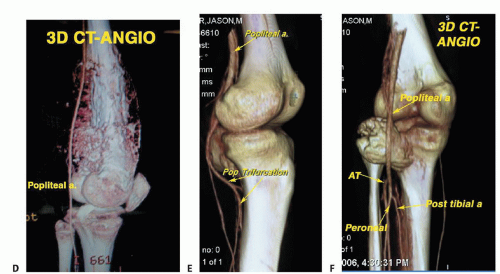
Angiography or three-dimensional CT angiography can be used to evaluate the superficial femoral and popliteal arteries. This is especially important if there is a large posterior
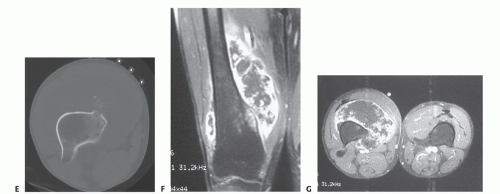
or medial extraosseous component. The late arterial phase of the angiogram or venous phase will show residual tumor blush. The degree of remaining vascularity correlates well with the tumor necrosis (FIG 5A,B). An unresponsive tumor as shown by a tumor blush requires a wider margin than a good responder (no tumor blush). More recently, three-dimensional CT angiography has replaced traditional angiograms; it shows the vascular anatomy well (FIG 5C-F).
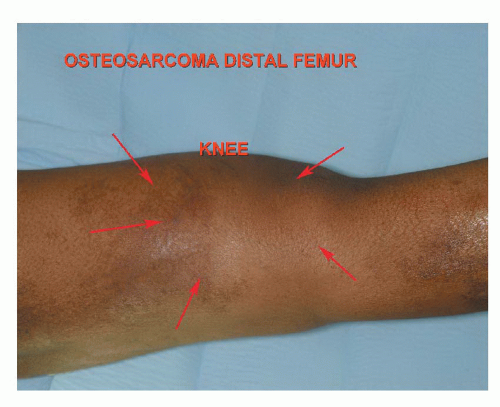
FIG 3 • Clinical photograph of an osteosarcoma of the distal femur. There is a large soft tissue mass (arrows). Ninety-five percent of all osteosarcomas have an extraosseous component.
Together, these studies help to determine the resectability of the tumor as well as the desired level of the femoral osteotomy.
A comprehensive knowledge of prosthetic stem lengths and widths is necessary to be sure that adequate proximal bone stock is available to proceed with endoprosthetic reconstruction.
SURGICAL MANAGEMENT
Surgical guidelines for limb-sparing surgery are as follows:
The major neurovascular bundle (popliteal vessels) must be free of tumor.
The resection of the affected bone should leave a wide margin and a normal muscle cuff (ie, 1 to 2 cm) in all directions (FIG 6).
All previous biopsy sites and all potentially contaminated tissues should be removed en bloc. All needle biopsy tracts must be removed (FIG 7A).
To avoid intraosseous tumor extension, bone should be resected 3 to 5 cm beyond abnormal uptake, as determined by preoperative studies.
The adjacent joint and joint capsule should be resected.
Adequate motor reconstruction must be accomplished by regional muscle transfers.
Adequate soft tissue coverage is needed to decrease the risk of skin flap necrosis and secondary infection. A medial gastrocnemius rotation flap provides excellent coverage of the prosthesis when required.
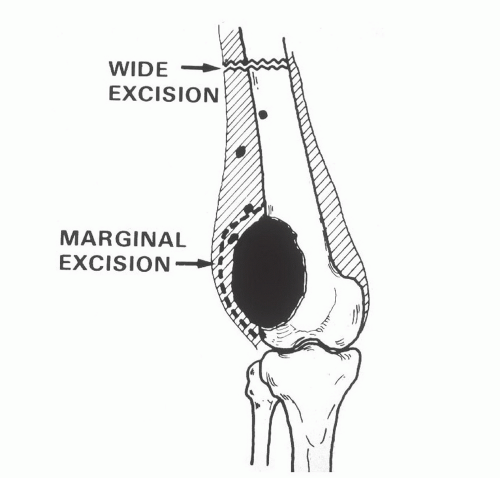
FIG 6 • Primary distal femoral osteosarcoma: soft tissue resection. The small black dots represent potential skin metastases. The planes of a marginal excision and a wide excision are shown.
Careful attention to the patient’s general condition is critical in the timing of limb-sparing procedures in cancer patients. Patients undergoing preoperative chemotherapy (FIG 7B,C) and radiation therapy (Ewing sarcoma) need an adequate hiatus before surgery. In general, surgery can proceed 2 to 3 weeks after these treatments are completed. The white blood cell count and platelet count need to be within a safe range and rising, and the skin must have recovered from the effects of radiation and must be nonerythematous.
When the procedure is used for a salvage reconstruction after failed internal fixation, failed total joint arthroplasty, or allograft procedures, a past history of infection can bode poorly if it is not completely eradicated before surgery.
Preoperative Planning
The intended level of resection should be determined before the patient comes to the operating room. A careful review of the diagnostic tests should confirm this location and should confirm that there is adequate bone stock left to accept the femoral stem in terms of both length and width. The distal femur should be resected with a safe oncologic margin (3 to 4 cm of normal marrow). The extremity lengths should be equal to within millimeters. To achieve this, intraoperative marks and measurements are made to ensure that the length before resection equals the reconstruction length.
When planning the primary resection and reconstruction, the surgeon should also be planning an amputation or revision.9 Ideally, the level of amputation should be at the same level it would have been had amputation been chosen as the original procedure to achieve local control. The surgeon should plan how he or she will revise this reconstruction in the event of infection or mechanical failure. The real goal
would be to retain the patient’s own hip and not go to a total femur replacement unless necessary, as this requires rehabilitating two joints in series, which is always a greater challenge for the patient.
Positioning
In the preoperative area or as anesthesia is being induced, the patient is given intravenous antibiotics. One gram of vancomycin is slowly infused over 1 hour, and this is repeated every 12 hours until the drains are removed. A single 80-mg dose of gentamicin or tobramycin is also given. An epidural catheter is routinely used for postoperative pain management.
After anesthesia is induced, a urinary catheter is placed. For the medial approach, the patient is placed in the supine position with the entire leg, including the inguinal area, prepared. This provides adequate access to the proximal femoral vessels.
A tourniquet is not used. A folded sheet placed transversely under the sacrum can elevate the pelvis to permit better access for draping. If the lateral approach is used, then the patient is placed in the lateral decubitus position on a beanbag with an axillary roll. A standard 10-minute preparation is performed, generally iodine-based.
Approach
The preferred approach is a medial longitudinal approach with exploration of the superficial femoral and popliteal vessels. All vessels that feed the tumor and distal femur are tied off (FIG 8). A lateral approach is used only when access to the proximal femur is needed for cross-pin stem fixation or when little residual proximal femur remains.
TECHNIQUES
▪ Resection and Reconstruction of the Distal Femur through a Longitudinal Medial Approach and Preparation for Cementing the Tibia, Patella, and Femoral Components
Position and Dissection
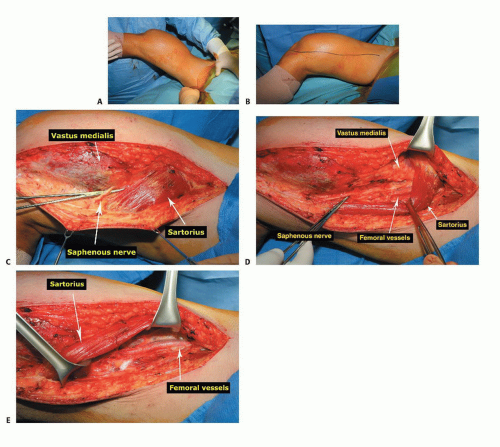
The patient is in supine position with the leg and inguinal area prepared out (TECH FIG 1A).
The incision is longitudinal, following the sartorius muscle from proximally in the thigh distal to beyond the tibial tubercle (TECH FIG 1B).
Any biopsy tract should be kept in continuity with the underlying tumor. Because the routine approach for primary tumors is medial, lateral or anterior open biopsy tracts need to be ellipsed and kept in continuity with the underlying tumor.
The saphenous nerve is identified and protected (TECH FIG 1C).
The interval between the sartorius and the vastus medialis is opened, exposing the superficial femoral artery and vein along with the saphenous nerve (TECH FIG 1D,E).
The vessels and the saphenous nerve are dissected from proximal to distal and are reflected posterior and medial along with the sartorius muscle.
All vessels (geniculates) are tied off with 2-0 or 3-0 silk ties as they course from the vessels toward the distal femur and tumor (TECH FIG 1F). The surgeon must not ligate the medial or lateral sural vessels, which are the main blood supply to the respective gastrocnemius muscles. These vessels are the basis of a gastrocnemius flap if required.
The surgeon should be careful at the canal of Hunter because the vessels are just deep to the adductor tendon.
Distal to the canal of Hunter, the popliteal vessels are dissected free and reflected posterior and medially (TECH FIG 1G). The short head of the biceps muscle is now seen coursing proximal to distal to join the long head laterally in the thigh.
The sciatic nerve is exposed and protected.
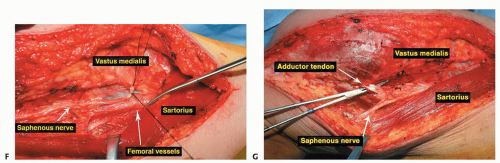
Proximal and medial in the thigh above the tumor, the junction between the adductors and vastus medialis can be opened to the femur to reflect the quadriceps laterally off the femur (TECH FIG 2A).
Deep to the medial intermuscular septum is the terminal profunda artery and vein, which may be ligated.
The superficial femoral vessels, along with the saphenous nerve and popliteal vessels, are dissected free from the tumor throughout its length to below the joint line (TECH FIG 2B,C).
The medial gastrocnemius muscle can be incised. The surgeon must not ligate the medial sural vessels (TECH FIG 2D,E).
With the femoral vessels completely dissected and reflected, the quadriceps or a portion of it, along with the patella and patellar tendon, are now reflected over the tumor, leaving the vastus intermedialis as a very satisfactory oncologic margin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree