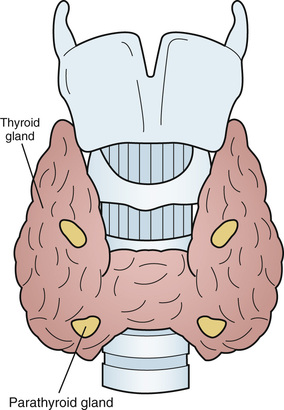
Jane Turton, Michael Stone, Duncan Cole In most individuals there are just four parathyroid glands, which are usually located at the superior and inferior poles of the thyroid gland and which arise from the third and fourth pharyngeal pouches during the fifth and sixth weeks of fetal development1 (Figure 89-1). A parathyroid gland is oval, is yellow in color, measures about 6 mm in a craniocaudal direction, and usually weighs less than 35 mg. Within a gland, there are four cell types: the chief cells, clear cells, oxyphil cells, and adipose cells.2 Parathyroid hormone (PTH) is produced by the chief cells and is an 84–amino acid polypeptide with biologic activity in the N-terminal portion of the molecule. It is synthesized as pre-pro-PTH, and is then sequentially processed to pro-PTH and finally to intact PTH (1-84). PTH is secreted as intact PTH and also in the cleaved C-terminal PTH form (C-PTH). The proportion of C-PTH secreted relative to intact PTH changes in response to circulating free calcium concentration. Intact PTH has a half-life of less than 5 minutes. It is metabolized by Kupffer cells in the liver, resulting in cleavage of its N-terminal portion and the intracellular degradation of the N-terminal fragment. C-terminal fragments are released back into the circulation from the liver. These fragments are cleared by the kidneys and may be elevated in patients with renal failure. C-terminal fragments have a longer half-life than the intact hormone and constitute the majority of circulating PTH forms. PTH secretion exhibits both a circadian rhythm, with a peak in the early hours of the morning and nadir in late morning/early afternoon,3 and a seasonal variation, being highest in the winter and lowest in the summer.4 The secretion of PTH occurs predominantly in response to a fall in extracellular free calcium concentration, which is sensed by the calcium sensing receptor (CaSR) on parathyroid chief cells. The CaSR, is a G protein–coupled receptor, found in the intestine, kidney, thyroid C cells, brain, and bone where it is involved in local calcium homeostatic processes.5 The gene mutation of the CaSR in familial benign hypocalciuric hypercalcemia results in an alteration of the calcium homeostatic set point.6 The secretion of PTH is also influenced by circulating levels of 1,25-dihydroxyvitamin D [1,25(OH)2D3], phosphate, and magnesium. 1,25(OH)2D3 suppresses PTH secretion by inhibiting gene transcription. Hyperphosphatemia increases PTH synthesis and secretion; the converse holds for hypophosphatemia. In states of significant chronic magnesium deficiency, PTH secretion is impaired; however, an acute magnesium deficiency can increase PTH secretion. In the kidney, PTH stimulates 1α-hydroxylation of 25-hydroxyvitamin D3 [25(OH)D3] to produce the biologically active 1,25(OH)2D3, promotes calcium reabsorption in the distal convoluted tubule, and inhibits the reabsorption of phosphate from the proximal tubule. In the small intestine, PTH has a small direct local effect, and circulating 1,25(OH)2D3 produced in the kidney promotes gastrointestinal absorption of calcium and phosphate.7 PTH increases osteoclastic resorption of bone at cortical surfaces and causes the release of calcium into the extracellular fluid. The net result of PTH action is an increase in extracellular calcium levels and a reduction in phosphate levels. In older adults, average PTH levels can be up to 35% higher than those measured in a young adult population.8,9 The cause of this includes the following factors: • A fall in 1,25(OH)2D3 production by the aging kidney as glomerular filtration rate falls from 125 mL/min at age 20 years to 60 mL/min at age 8010 • A reduction in the responsiveness of renal 1α-hydroxylase to circulating PTH11 • A reduction in dietary intake and absorption of calcium and vitamin D12 • A reduction in vitamin D production in the skin as a result of less frequent and less efficient sunlight exposure. Individuals who are housebound or living in nursing homes are at particular risk of developing abnormal PTH secretion secondary to vitamin D deficiency.13–16 Secondary hyperparathyroidism is the most common disorder of calcium homeostasis in older adults and has a prevalence of between 20% and 60% in the population older than 75 years.17 Secondary hyperparathyroidism occurs when there is vitamin D deficiency or insufficiency, and the resultant decrease in serum calcium causes a reduction in the expression of the CaSR in the parathyroid glands, leading to parathyroid gland hyperplasia, which in turn causes a physiologically appropriate increase in PTH production. The combination of vitamin D deficiency and secondary hyperparathyroidism is associated with an increased risk of fracture.17,18 There is an associated increase in mortality from cardiovascular disease19 independent of bone mass, serum 25(OH)D, and renal function.20–22 The first-line treatment for secondary hyperparathyroidism is a combined preparation of elemental calcium 1000 to 1200 mg and vitamin D3 20 µg (800 IU) daily. Chapuy and colleagues showed that reducing PTH levels and improving serum 25(OH)D3 levels using this combination could reduce risk of hip fracture by 30% at 18 months and that this efficacy was maintained out to 3 years.23 If for any reason a patient cannot or will not adhere to this regime, vitamin D2 or D3 alone, 1α-hydroxycholecalciferol, or 1,25(OH)2D3 can be used. 1,25(OH)2D3 is particularly useful for patients with renal failure, although careful monitoring of serum calcium is required. The biochemical presentation of primary hyperparathyroidism is a raised plasma PTH and hypercalcemia. The peak incidence occurs between ages 55 and 64 years at 1 : 1000. The prevalence is 2% in persons older than 75 years, and it is three times as common in women as in men.24 Primary hyperparathyroidism is caused by a single adenoma in 80% of cases, four-gland hyperplasia in 15% of cases, and carcinoma in less than 1% of cases.25 A rare but notable form of hypercalcemia that can mimic primary hyperparathyroidism is benign familial hypocalciuric hypercalcemia. Changes in the levels of circulating PTH have been shown to have an effect in bone, increasing the bone remodeling space. In trabecular bone, high levels of PTH increase bone formation. However, in cortical bone, high levels of PTH increase bone loss, cause a fall in bone mineral density (BMD), and increase the risk of fracture independent of 25(OH)D3 levels.26–28
Disorders of the Parathyroid Glands
Introduction
Physiology of Parathyroid Hormone
Calcium Homeostasis and Changes with Age
Secondary Hyperparathyroidism
Primary Hyperparathyroidism
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Disorders of the Parathyroid Glands
89






