Barbara Weinstein
Disorders of Hearing
A guiding principle for the care of older adults is the delivery of person-centered care (PCC), with patient preferences elicited and incorporated into medical decision making. A cornerstone of PCC is communication wherein individuals can make themselves understood and understand what others are saying. When older adults are unable to communicate because of untreated or unrecognized hearing loss (HL), the health consequences are dramatic, especially among those with multimorbidity. Ironically, HL is considered a little recognized consequence of aging, despite the physical, psychosocial, and cognitive correlates of this unavoidable aspect of aging. With Americans living longer and functional status and remaining life expectancy emerging as important prognostic indicators, an understanding of HL and its prevalence, cause, consequences, and treatment are crucial to optimization of therapies and care planning.
Life expectancies at ages 65 and 85 years have increased dramatically, bringing with it an increase in the amount of time spent in all major activities, including work and retirement. Older adults are spending an increasing proportion of their life in retirement, so the ability to communicate effectively takes on even greater importance with these retirement and work force trends. As people age, the likelihood of experiencing one or more chronic conditions increases. Over 50% of older adults have three or more chronic diseases, typically referred to as multimorbidity. Coincident with increases in life expectancy, the number of older adults with multimorbidity has been increasing dramatically. Communicating with the patient about clinical management options within the context of risks, burdens, benefits, time horizon to benefit, import of adherence, and prognosis (e.g., functional status, quality of life) is key to the delivery of quality care.
Considered a geriatric syndrome, age-related hearing loss (ARHL) is a major public health problem and a contributor to the global burden of disease.1 Hearing impairment is one physical disability that is increasing in prevalence in society in general and in older adults in particular. Globally, adult-onset HL is the second leading cause of years living with disability (YLD) behind depression and is a larger nonfatal burden than alcohol use, osteoarthritis, and schizophrenia.2 Over the last generation, the HL population has grown at a rate 1.6 times that of U.S. population growth, with 36 million Americans self-reporting hearing impairment. By the year 2030, at least 21 million Americans older than 65 years are projected to have a hearing impairment. HL prevalence ranges from 30% to 47% among persons older than 65 years, doubling with each age decade, so that nearly two thirds of persons 70 years of age and older and 80% of persons older than 85 years having a HL that affects their communication ability.3 The risk of experiencing a HL increases dramatically with each additional decade, with rates of decline more accelerated for the oldest old cohort and with 50% having at least a moderate HL.1 Mean hearing levels of persons 95 years of age and older are consistent with moderately severe to profound HL, significantly poorer than the cohort between 80 and 94 years. The high prevalence and severity of hearing impairment among the oldest old has will affect transitions in care, palliative care, and home care.
Hearing impairment, increasing age, and male gender are the most relevant risk factors for tinnitus, with 11% of adults suffering from ARHL experiencing permanent and persistent tinnitus.4,5 Because the inner ear subserves the sense of hearing and balance, age and high-frequency HL are risk factors for vestibular dysfunction, which is highly prevalent among older adults. Prevalence ranges from 69% among 70- to 79-year-olds to 85% among those older than 80 years.6 The sensation of dizziness is highly prevalent among people older than 65 years, accounting for 8 million primary care physician visits in the United States. Among those 70 years of age and older, high-frequency HL is associated with reduced saccular function, with age and noise exposure significantly associated with cochlear and saccular dysfunction.7 Older adults with chronic dizziness or imbalance are two to three times more likely to fall in comparison with older adults who do not experience these problems. Finally, population-based studies of persons older than 50 years have revealed a prevalence of between 5% to 10% of persons with objectively measured concurrent hearing and visual problems; this is termed dual sensory impairment (DSI).8 DSI increases in prevalence with age, so that the oldest old are at greatest risk for DSI, with woman having a slightly greater prevalence than men. The likelihood of comorbid and secondary conditions is greater in persons with DSI than in those with a hearing or vision problem.9 DSI is associated with mortality, especially among older adults with concurrent moderate to severe HL and any presenting or best-corrected vision loss.10
Modifiable and Nonmodifiable Risk Factors for Hearing Loss
ARHL is a multifactorial condition with a number of modifiable and nonmodifiable risk factors.11 Nonmodifiable factors include increasing age, genetic predisposition, race (decreased risk in African Americans), and gender (males, increased risk).11 Modifiable risk factors include environmental exposure (e.g., noise, ototoxicity), smoking, and multiple health comorbidities, including cerebrovascular disease, cardiovascular disease (CVD), and diabetes. Cognitive decline increases the risk for hearing impairment, and kidney disease, metabolic conditions such as lupus, thyroid dysfunction, and head trauma are also medical conditions associated with HL. Alcohol consumption in moderation appears to be a buffer against developing HL. When present in older adults with chronic conditions ranging from CVD to diabetes and falls, HL is likely to increase the burden of these problems. Data from the Health ABC Study, a population-based prospective cohort study, has revealed that history of smoking is associated with poorer high-frequency hearing levels in men.12 Similarly, data from the NHANES cross-sectional survey has confirmed that heavy smoking increases the odds of HL nearly twofold.13 Exposure to loud noise accelerates age-related HL, and noise exposure and history of CVD appear to have a synergistic effect, elevating hearing threshold levels.13
CVD risk factors, including higher levels of triglycerides and poorer resting heart rate, are related to poorer hearing.12 This association is likely linked to the fact that an insufficient cochlear blood supply can disrupt the chemical balance of the fluids within the inner ear, influencing the activity of the hair cells and activation of the auditory nerve.12 Similarly, a history of diabetes mellitus is linked to poorer hearing sensitivity, most likely due to the effect on the cochlear vascular system, with a prevalence higher among persons with diabetes as compared to those without.13,14
Adverse drug reactions (ADRs) in the form of auditory or vestibular symptoms are prevalent and severe among older adults. ADRs occur for a variety of reasons, including lack of compliance due to compromised understanding of the prescription. This could be attributable to hearing or visual problems or possibly because of confusion between two medications that might sound alike (e.g., Plavix or Paxil) to a hearing-impaired person or may look alike to a visually impaired older adult. Some of the auditory or vestibular side effects of medications include dizziness, ear discomfort, lightheadedness, vertigo, bilateral HL that is often profound and delayed in onset, and tinnitus. The severity and time of onset of is dose-dependent and typically rapid in onset, occurring soon after the drug is administered. Although symptoms may appear months after the medication has been administered, the effects often progress for several months after cessation of the medication, especially in the case of chemotherapeutic agents. Older adults with HL prior to chemotherapy with cisplatin are more likely to experience a threshold shift following administration of the drug, and close monitoring and counseling are recommended. Otoprotective medications should be considered as well. When aminoglycoside antibiotics are administered with loop diuretics, the ototoxic effect is synergistic and powerful. Symptoms of ototoxicity include development or intensification of tinnitus in one or both ears, appearance of a new sound in the ear different from already existing tinnitus, fullness or pressure in the ears (i.e., different from that caused by infection), progression of an already existing HL, and development of a spinning sensation aggravated by motion, which might be accompanied by nausea. Ototoxic medications include but are not limited to the following: aminoglycoside antibiotics, chemotherapeutic agents, including cyclophosphamide, carboplatin, cisplatin, loop diuretics, and aspirin or salicylate-containing medications.
Cognitive impairment increases the risk for HL, and HL increases the risk of developing cognitive impairment.1 Predictors of rate of change in hearing levels over an 11-year time frame include baseline age, gender, and probable cognitive impairment. Probable cognitive impairment (Mini Mental State Examination [MMSE] score ≤ 23) at baseline is associated with faster rates of change in hearing levels and poorer initial hearing levels at baseline.1 Greater rates of change in hearing levels take place in persons with clinically diagnosed hypertension at baseline. The incidence of cognitive impairment is also associated with poorer hearing levels.1 HL is independently associated with all-cause dementia, and the risk of all-cause dementia increases linearly with HL severity.15 The mechanisms for the observed association have not yet been determined, but researchers have speculated about a possible common neuropathologic process or via the pathway of reduced social network size, diminished quality interpersonal relations, and social isolation, which is linked to hearing impairment.
The Aging Auditory Mechanism
The auditory system is an integrated system involving an interplay among its many components, including the outer, middle, and inner ears (peripheral auditory system) and the brain (central auditory system).16 An impoverished output from the peripheral auditory system due in part to age-related changes affects the integrity of the input to the central auditory system and ultimately the communication challenges associated with ARHL. The lack of uniformity in pathologic and physiologic changes across individuals may help explain e individual differences in speech understanding in a challenging acoustic environment, which is the hallmark of ARHL.
Age-related changes in the outer and middle ears have few implications for communication ability. Increased activity of cerumen glands in the cartilaginous portion, physical obstruction due to a hearing aid, frequent use of cotton-tipped swabs, or production of drier and less viscous cerumen contribute to the excessive accumulation of wax to which older adults are susceptible. One of the most common reasons for physician visits is accumulation of cerumen because of failure of the self-cleaning mechanism. Accumulation of excessive cerumen (cerumen impaction) is present in approximately one third of older adults, with estimates ranging from 19% to 65%. It is more common in older adults, nursing home residents, and persons with cognitive impairment. The primary sequela of impacted cerumen is HL, which typically produces noticeable improvements in hearing and understanding following treatment. Curettage and irrigation are the two approaches to removing cerumen in primary care, and each is associated with risks and benefits. In diabetics or immunocompromised patients, for example, management can pose problems for primary care physicians.
The middle ear is susceptible to minor age-related changes that have little impact on hearing, whereas the site of conversion of mechanical energy to an electrophysiologic signal, the inner ear, is composed of several functional components vulnerable to the effects of aging. The organ of Corti is the structure most susceptible to age-related changes that ultimately interfere with the transduction process integral to the reception of sound. Historically ARHL has been classified into three types—sensory, neuronal, or metabolic.11
The primary histopathologic changes in the organ of Corti include loss of hair cells beginning in the extreme basal end, greatest in persons older than 70 years and most pronounced for outer hair cells (OHCs). It is now well accepted that changes in OHCs are due in large part to noise trauma rather than age. The resulting HL is primarily for high-frequency sounds (e.g., s, sh, th), which are processed in the basal end of the cochlea. Neuronal pathology tends to manifest as loss of spiral ganglion neurons and is diffuse, involving all three turns of the cochlea and resulting in considerable difficulty understanding speech. The deleterious effects of aging are typically first seen in highly metabolic tissues in the body, and the most prominent feature of age-related HL of the metabolic variety is atrophy of the stria vascularis, an area very high in metabolic activity.17 The most common feature of ARHL in the periphery is degeneration of the stria vascularis. Loss of function of the cells in the stria vascularis and/or spiral ligament appears to result in disruption of inner ear ion homeostasis, thereby causing a decline in endocochlear potential (EP).11 When strial degeneration exceeds 50%, EP values drop rather substantially.18
A hallmark of ARHL is neuronal loss in the periphery, which may begin at any age. The neuronal changes which tend to impact processing of speech sounds include the following: (1) disrupted neural synchrony, which is associated with reduced amplitude of the action potential; (2) decreased neural inhibition; (3) longer neural recovery time; (4) a decrease in the number of neurons in the auditory nuclei; (5) changes in synapses between inner hair cells and the auditory nerve; and (6) age-related changes in the level of inhibitory neurotransmitters.19,20 The overall loss of neurons and loss of acoustic nerve activity interfere with temporal resolving abilities, which contributes in large part to the auditory processing and speech understanding problems experienced by many older adults.21 Impoverished auditory signals and reduced stimulation from the impaired cochlea disrupt the tonotopic organization throughout the central auditory system, including the cochlear nucleus, inferior colliculus, and midbrain, precipitating changes in cortical reorganization and brain morphometry.22 Specifically, decreased acoustic input associated with age-related changes in the auditory periphery is associated with a selective downregulation of normal adult inhibitory γ-aminobutyric acid (GABA)–ergic function in the inferior colliculus (IC). Decreased acoustic input from the auditory periphery is associated with significant changes in GABA neurotransmission in the normal adult IC. Central auditory reorganization due to plasticity does take place, so that intact regions of the tonotopic map adjacent to the impaired regions tend to become responsive, confirming an auditory reorganization.
Age-related changes also take place in the temporal lobe of the aging brain. Recent neuuroimaging studies have demonstrated an independent association of hearing impairment with reduced cortical volumes in the auditory cortex, as well as accelerated rates of atrophy in the lateral temporal lobe and whole brain.22,23 Regarding the latter, magnetic resonance imaging (MRI) studies have revealed that persons with hearing impairment have significantly more shrinkage (i.e., specific volume declines) in the structures responsible for processing speech information—namely, the superior, middle and inferior temporal gyri.22 The temporal region of the auditory cortex is involved in processing spoken language and is integral to linguistic, cognitive, and speech processing in challenging situations, such as semantic memory and sensory integration.23
Behavioral Implications of Anatomic and Physiologic Changes
The classic complaint of older adults with ARHL—“I can hear people talking but cannot understand what they are saying, especially in noisy situations”—aptly describes the problems resulting from the reduction in transmission, reception, and perception of the speech signal attributable to sensorineural HL. ARHL (presbycusis) is sensorineural, characterized by a loss of acuity and loss of clarity (distortions). The attenuation or loss of audibility for low- and high-frequency sounds interferes with the detection of warning signals and with speech understanding. The distortion component of sensorineural HL is associated with reductions in spectral and frequency resolution, which further compromise speech understanding. Specifically, older adults have difficulty comprehending the following: (1) speech in quiet and noisy situations; (2) speech when the rate of speaking is fast; (3) accented speech; (4) speech spoken from a distance; and (5) when multiple talkers are speaking. Characteristics of the speaker’s voice, complexity of the message, listener’s knowledge of the language, use of gestures, and availability of contextual information also influence speech understanding. Speech understanding is compromised when presented without the benefit of contextual information, when multiple speakers are talking, and when the speaker is not nearby. Communicating in challenging situations demands the expenditure of mental effort. making it an effortful and fatiguing process.24 Cognitive processing (e.g., working memory, speed of information processing, divided attention), which also declines with age, is crucial to the functions of listening and comprehending, further compromising communication ability.24 Finally, the advantages of binaural listening, including directional hearing, are reduced in older listeners, potentially compromising their safety. Brain plasticity enables compensation when older adults can use knowledge and context to advantage during listening. Although many aspects of cognitive processing that are important to speech understanding decline with age, knowledge and use of context are well preserved and can enhance recall, comprehension, and communication.
Consequences of Age-Related Hearing Impairment
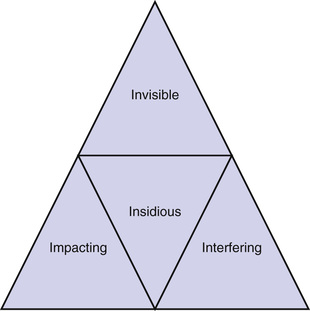
The behavioral implications of hearing and speech understanding difficulties that characterize older adults are considerable. The myth that HL is harmless has been debunked, and it is becoming increasingly clear that when untreated, HL can be costly to the individual and family members. The unique features of ARHL can be summarized using the four Is, as shown in Figure 96-1. Considered an invisible handicap, ARHL is (1) insidious, developing gradually so that the individual does not notice or admit to HL until 7 to 10 years after it typically begins to set in; as the HL progressively worsens, the individual’s personality, behaviors, and outlook toward life may change; (2) interfering, notably with communication and performance of many of the instrumental and routine activities of daily living; (3) impacting on self-esteem, sociability, and quality of life, if left untreated; and (4) invisible, so people adapt by using avoidance behaviors that enable them to get by in many situations.
Given the insidious and invisible nature of ARHL, it is not surprising that its consequences interfere with many facets of daily life, posing a threat to healthy and active aging. The incidence of depressive symptoms is higher among persons self-reporting a hearing handicap as compared to those without, and self-reported hearing handicap is an independent predictor of depressive symptoms.25,26 Furthermore, the prevalence of moderate to severe depression (Patient Health Questionnaire [PHQ-9] score = 10) is dose-dependent, with 4.9% of individuals reporting excellent hearing, 7.1% good hearing, and 11.4% reporting a little trouble, or greater HL.27 HL and a self-reported hearing handicap are associated with perceived social isolation for those with moderately severe to severe HL, and a person with a significant self-reported hearing handicap is at greatest risk for being subjectively and objectively socially isolated.
HL may be mechanistically associated with cognitive decline through social isolation.28 HL is independently associated with accelerated cognitive decline and incident cognitive impairment in community-dwelling older adults. Individuals having HL were found to have a 30% to 40% accelerated rate of cognitive decline and a 24% increased risk for incident cognitive impairment over a 6-year time period as compared with individuals having normal hearing.28 Compared to individuals with normal hearing, individuals with a mild, moderate, or severe hearing impairment, respectively, have a twofold, threefold, or fivefold increased risk of incident all-cause dementia over more than 10 years of follow-up.29
Additionally, increased severity of HL is associated with impaired activities of daily living (ADLs) and instrumental ADLs; those with moderate to severe HL have higher odds of having difficulty with ADLs as compared to those without HL.26 Older adults with HL have increased use of community support and informal support networks. Use patterns are related to HL severity, with those with moderate to severe HL at the greatest risk of developing reliance on community support systems.30 The link between HL and independence is highlighted by the fact that persons with baseline HL were found to have a higher likelihood of relying on support systems than those with normal hearing at 5-year follow-up.30 The prevalence of falls increases with age and is highest among those in poor health, those with two or more functional limitations, and those with HL, visual loss, and depression.31 Persons with hip fractures have a higher prevalence of hearing impairment, followed by vision impairments and dual sensory impairment.32 The latter findings are not surprising, given the fact that age-related vestibular abnormalities appear to be bilateral and to increase in prevalence with advancing age.33 There is a decline in saccular function with age, which has been correlated with HL in the higher frequencies.7 The more severe the high-frequency HL, the greater the decline in saccular function. Older women with poor hearing threshold levels had an higher risk for falls than older woman with essentially normal hearing acuity.34 The proportion of participants with two or more falls was 30% in those with the poorest hearing levels and 17% in the group with the best hearing levels. In a subsequent study, it was reported that most older woman in the sample reporting walking difficulties expressed a fear of falling (FOF), and persons reporting FOF more often reported balance, vision, and hearing difficulties.35 The presence of multiple, coexisting, self-reported sensory difficulties substantially increased the risk of mobility decline, leading the authors to speculate that FOF may serve as an exacerbating behavioral factor, increasing mobility decline in persons with sensory impairments.
Anatomically, the link between HL, mobility limitations, and falls makes sense because the hearing and vestibular organs share fluid-filled bony compartments and blood circulation and have similar mechanosensory receptor hair cells, which detect sound, head movements, and orientation in space.34 An additional explanation may be related to cognitive load. Older adults with impaired hearing must allocate a greater proportion of their attention to maintaining their balance during daily activities, including walking and talking. Impaired hearing may “place additional demands on attention sharing and thus further increase fall risk.” 34 Among persons 60 years of age and older, ambulation and hearing were predictive of mortality.35 Disability in hearing showed a stepwise and strong impact on mortality, and disability in ambulation was associated with a stepwise increase in mortality. There exists a link between hearing impairment and mortality risk which was mediated by falls, poor self-rated health, and poor cognitive status (MMSE score ≤ 24).36 Compared with participants having normal hearing, those with HL at baseline were more likely to be male, older, cognitively impaired, diabetic, and underweight and more likely to have a self-reported history of angina, myocardial infarction, stroke, low self-rated health, and observed difficulty in walking or use of walking aids. Using a structural equation modeling (SEM) pathway analysis to model the relationship over a period of 5 years between hearing impairment, mortality, and covariables, a significant association emerged between hearing impairment and mortality after adjusting for confounders such as age and gender. There was no gradient effect from the severity of HL and mortality risk.
Another possible explanation for the link among falls, mobility restrictions, and HL is that FOF or a history of falls may decrease participation in various activities, which in turn may accelerate the disablement process, thereby increasing risk of falling.34 There is a connection between self-reported and objectively measured physical activity levels and HL.37 Participants with moderate HL or greater had a greater odds of reduced self-reported physical activity and less accelerometer-measured activity than individuals with normal hearing. Reduced physical activity levels is one of the criteria that characterizes frailty or low physiologic reserve and vulnerability to stressors.38 Unintentional weight loss, slow walking speed, weakness and exhaustion are also classic signs of frailty. Using data on individuals 70 years of age and older, the 1999 to 2002 cycle of the National Health and Nutrition Examination Survey (NHANES) self-reported HL was independently associated with frailty in women.39 Because frailty is associated with hospitalization, it is of interest that HL is also associated with burden of disease and odds of hospitalization, health care use, and health care expenditures. The literature linking mortality risk, mobility limitations, reduced physical activity levels, and HL underscores the need to screen the hearing of patients at risk for falls or those having a history of falls; perhaps treating hearing impairment via one of the interventions along the continuum can be effective in reducing mortality risk.32,36
HL is independently associated with increased burden of disease, poorer self-reported health, history of CVD, and increased rates of hospitalization and health care use.40,41 Longitudinal studies have demonstrated that the physical composite score and mean scores for seven of the eight SF-36 domains were significantly lower at the 10-year follow-up among participants with self-reported hearing handicap at baseline. Furthermore, the greater the degree of HL, the higher the likelihood of experiencing functional disability, with those with moderate to severe HL at greatest risk. This finding is not surprising because communication is integral to performing routine instrumental ADL tasks, such as talking on the telephone and shopping. The presence of distracters is in part responsible for the latter associations. Similarly, driving performance is affected by HL, especially in the presence of visual and auditory distractions.42 Older drivers with moderate to severe hearing impairment demonstrate worse driving performance in the presence of distracters as compared to those with normal to mild hearing impairment. Hence, the use of GPS systems, speaking on the telephone, conversing with other passengers, or listening to the radio could create in-car distractions, which may diminish driving performance. This finding is consistent with the so-called effortfulness hypothesis, which holds that the extra effort associated with listening to and understanding a degraded auditory signal consistent with ARHL takes resources from other cognitive processes. Because the typical HL characterizing the oldest old tends to be moderate to severe, these individuals are at greatest risk for many of the negative consequences of HL (Box 96-1). Based on a data set of over 600,000 Australians, it appears that at each stage of advancing HL and resultant disability, there was a change in health status.43 Notably, at the point of severe and profound self-reported hearing disability, the decline in health status is most pronounced. When asked to rate the disability that caused the most disability, respondents rated HL as the third most problematic condition after chronic pain and restrictions in physical activity.








