Chapter 18
Disorders of Carbohydrate Metabolism
Marjorie Dixon, Anita MacDonald, Jacky Stafford, Fiona White and Pat Portnoi
An Introduction to Inherited Metabolic Disorders may be found at the beginning of Chapter 17.
Disorders of Galactose Metabolism
Anita MacDonald Pat Portnoi
Galactose is a monosaccharide that plays an important role in the biosynthesis of complex carbohydrates, glycoproteins and glycolipids [1]. There are three autosomal recessive disorders of galactose metabolism, with variable clinical phenotypes, caused by one of three sequential enzymes in the Leloir pathway: (i) galactokinase, (ii) galactose-1-phosphate uridyl transferase (GALT) and (iii) uridine diphosphate galactose-4-epimerase (GALE), which result in the inability to metabolise galactose (Fig. 18.1).
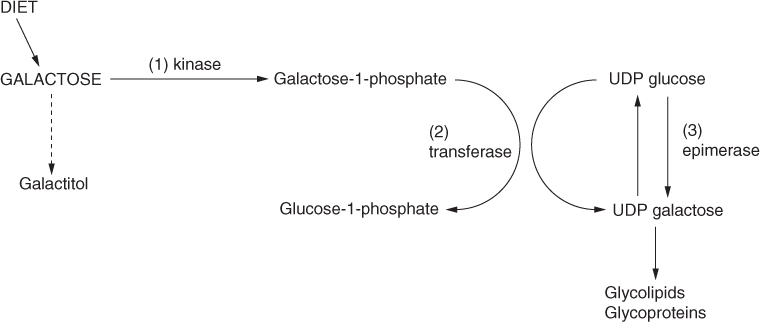
Figure 18.1 Pathways of galactose metabolism.
Galactosaemia
Introduction
Galactosaemia is a multi-organ disorder with wide clinical variability and over 220 different mutations or polymorphisms have been identified [2]. Abnormal glycosylation of glycoproteins and glycolipids may occur [3]. Direct biochemical consequences of GALT deficiency include accumulation of galactose-1-phosphate which, in turn, is metabolised to galactitol and galactonate, both of which accumulate in abnormal quantities in tissues [4]. Three forms of galactosaemia are described: (1) classical galactosaemia, (2) clinical variant galactosaemia and (3) biochemical variant galactosaemia [5].
Genetics, incidence and clinical features
The classical genotype is typified by Q188R/Q188R, the clinical variant by S135L/S135L and the biochemical variant by N314D/QI88R. The incidence in Western Europe is estimated to be between 1 in 16 000 to 1 in 40 000 [6–9]. It is particularly common in the traveller population of the Republic of Ireland where incidence is estimated to be 1 in 480 [10]. The most common mutation in Europe and North America is Q188R [11, 12].
Classical galactosaemia was first described in 1908 [13] and it has been treated by diet since 1935. Homozygosity for the Q188R mutation, associated with substantial or complete loss of GALT activity, causes markedly elevated erythrocyte galactose-1-phosphate concentrations at diagnosis. Many patients are at risk of lethal Escherichia coli sepsis, liver disease and cataracts in the neonatal period. In the long term, it is associated with cognitive impairment, language delay, speech deficits and premature ovarian insufficiency [14].
Patients with S135L/S135L may manifest with acute liver disease in childhood but may not show some of the other chronic complications of classical galactosaemia. This is the most frequent mutation in black Africans [15].
The mutation N314D (C940>G; Duarte-I-variant) leads to a residual GALT activity of about 25%. On a galactose containing diet galactose-1-phosphate concentrations are within target reference range, but there are increased concentrations of other metabolites. These concentrations appear to correlate with galactose intake and do not appear to cause clinical symptoms [16, 17] although long term outcome studies are not reported [5]. This variant of galactosaemia is commonly detected by newborn screening.
Clinical presentation
Classical galactosaemia presents in the neonatal period with life threatening illness after galactose is introduced in the diet. Symptoms and signs include poor feeding, vomiting and diarrhoea, weight loss, jaundice, hypotonia, cataracts, hepatosplenomegaly, hepatocellular insufficiency and encephalopathy [18]. Biochemical findings include abnormal liver function tests, abnormal clotting, hypoglycaemia, raised plasma amino acids and renal tubular dysfunction [19]. Rapid improvement occurs on stopping lactose containing feeds.
Cataracts may develop at the onset of symptoms and result from activation of the aldose reductase shunt, with consequent accumulation of galactitol in the crystalline lens [20]. Rarely, patients may present after the newborn period with faltering growth and developmental delay [15].
Long term outcome
Although serious long term complications may occur (Table 18.1), there is a wide variation in the extent of clinical problems between individual patients with no apparent relationship between the time of initiating treatment, severity of galactose restriction, treatment adherence or monitoring protocol [14]. However, verbal dyspraxia and premature ovarian failure are associated with homozygosity for the Q188R mutation [21, 22].
Table 18.1 Long term complications in galactosaemia
| Speech defect |
| Cognitive deficits |
| Learning problems |
| Cataracts |
| Hypergonadotrophic hypogonadism or primary ovarian insufficiency in females |
| Reduced bone mineral density |
| Growth disturbance |
| Cerebellar ataxia/tremor/dystonia |
Cognitive development, executive and neurological function
In many studies of cognitive development, verbal and performance IQ [12, 23–28], memory and executive functions were in the low average range [23], with some children attending special schools [29]. They are more likely to leave school with fewer educational qualifications [30] and less likely to be employed compared with the general population [31]. Nevertheless, there is a range of ability with some achieving university education and attaining higher degrees. Although a decline in intellectual performance with age has been described, evidence from a longitudinal childhood study suggested IQ remains unchanged [32]. Neurological or movement problems (tremor, extrapyramidal motor disturbances) may occur in up to 40% of patients [14]. Neuroimaging studies confirm poor myelination, scattered white matter abnormalities, cerebral atrophy and cerebellar atrophy, as well as abnormalities in glucose uptake and metabolism in many brain regions [33].
Speech
Delayed speech vocabulary and childhood apraxia of speech (CAS) are common [30, 34, 35]. In many cases, impaired speech is related to a decreased IQ [36]. CAS is a type of motor speech disorder characterised by inconsistent consonant and vowel errors, difficulty transitioning between articulatory movements and inappropriate prosody during speech [35].
Growth
Growth may be delayed in childhood and early adolescence, but final height is usually normal, although decreased height z-scores compared to mid-parental target height have been described [37]. There is also a decrease in fat mass and lean tissue mass compared with actual height z-scores [38].
Bone
Low bone mineral density is evident in male and female prepuburtal and postpubertal children and bone loss is common in adults [37, 39–41]. A correlation was found with the severity of osteopenia, patient age and use of hormonal supplementation, but the exact mechanism is not well understood. There is suggestion of an increase in bone turnover from childhood to adolescence with a possible imbalance between bone resorption and bone formation processes [42], whilst others have suggested a decrease in bone metabolism associated with a low insulin-like growth factor (IGF-1) z-score [37, 40]. Also there may be a possible intrinsic defect in the galactosylation of the collagen matrix of bone resulting in decreased mineralisation [39]. If bone mineral content is low, nutritional intake, physical activity and oestrogen supplementation should be optimised [3]. In a group of children and teenagers receiving a combination of calcium (750 mg/day), vitamin K (1 µg/day) and vitamin D3 (10 µg/day) there was significant improvement in bone formation markers and bone mineral content [43].
Fertility
Primary ovarian insufficiency (POI) occurs in 80% of women with classical galactosaemia despite treatment [18, 44, 45]; the aetiology is unknown. Most women develop primary or secondary amenorrhoea [46] and premature menopause usually occurs in the third decade [3, 47]. Clinical surveillance includes screening for abnormalities in ovarian function at an early age. Treatment consists of age appropriate oestrogen/progesterone supplementation.
Despite the high prevalence of POI several spontaneous pregnancies have been described [6, 48, 49], particularly in black women with milder mutations. Others have been reported in women homozygous for QI88R mutation [6, 49, 50]. Pubertal development and fertility are normal in boys, although low semen volume and higher than expected prevalence of cryptorchidism is reported [51].
Pregnancy in women with classical galactosaemia
Only a subtle and clinically insignificant increase of galactose metabolites occurs during pregnancy [52, 53] so no additional treatment is required during pregnancy for women with classical galactosaemia. Postpartum there is a transient increase in red blood cell metabolite concentrations and renal metabolite excretion. Normal healthy infant outcome is reported in the few pregnancies documented. A small number of women have successfully breast fed their infants with no adverse effect [52].
Quality of life
A lower health related quality of life has been reported in adults with galactosaemia. Significantly lower scores in cognitive and social domains occur [29], with social difficulties in at least 50% of patients [14]. In particular, the following are described: interpersonal problems, bullying [54], a lower number of friends, boys performing less competitive sports and socialising less [55]. Adults may have shy and timid personalities, being tense, overanxious or oversensitive with poor self-image. They are more likely to remain single [56], live with their parents [30], have delayed psychosexual development [31, 55] with men less likely to engage in sexual activities [47]. There is a twofold ‘odds’ increase of depression for each 10 year age increase in adults [47].
Endogenous production of galactose
Endogenous galactose is thought to originate from the transformation of glucose and from the recycling of glycosylated proteins and lipids [52]. It is considered to be a major cause of late complications [57]. Significant amounts of endogenous galactose is produced in patients with galactosaemia, release rates being several fold higher in infants than in adults [58] with adults producing approximately 13 mg/kg body weight/day compared with approximately 41 mg/kg body weight/day in newborns [59].
It is suggested that long term clinical abnormalities originate from foetal exposure to endogenously produced galactose or its metabolites in utero [32, 60, 61].
Diagnosis of galactosaemia
In many countries, galactosaemia is detected by neonatal screening, but diagnosis requires confirmation by a second independent method, e.g. determination of galactose-1-phosphate uridyl transferase activity in red blood cells or DNA analysis. In non screened populations, infants are diagnosed from clinical presentation. The Beutler test (a fluorescent spot test) may be used as an initial investigation and then diagnosis confirmed by further biochemical and/or molecular testing. Although newborn screening may be life saving through earlier identification it does not appear to change the incidence of long term complications [14].
If galactosaemia is suspected, a low galactose infant formula should be commenced immediately, even before diagnosis is confirmed.
Biochemical monitoring
There is no consensus as to the most relevant biomarker(s) for monitoring patients with galactosaemia. Measurement of red cell galactose-1-phosphate has been widely used to monitor dietary compliance. This is high at diagnosis and falls progressively after the introduction of dietary treatment but it may still take up to 1 year or even longer to decrease to the treatment reference range (laboratories use variable target ranges so a local treatment reference range should be used). Concentrations do not decrease to the levels found in normal, healthy individuals [3]. The units used to express galactose-1-phosphate concentrations vary between different laboratories, usually according to methodology [62].
Galactose-1-phosphate concentrations do not vary greatly in individual patients so frequent monitoring is not indicated [62] (Table 18.2). There is no correlation with clinical outcome [63]. Erythrocyte galactose-1-phosphate is commonly used as a measure of dietary adherence but does not appear to help identify mild dietary indiscretions. Other metabolites such as urinary galactitol and red cell galactonate have been considered. IgG N-glycan profiling may be a more sensitive and informative method of monitoring dietary adherence [63], although further investigation is needed.
Table 18.2 Suggested frequency of measuring galactose-1-phosphate concentrations [62]
| Age | Frequency of measuring galactose-1-phosphate concentrations |
| 0–1 year | Every 3 months |
| 1–14 years | Every 6 months |
| Over 14 years | Annually |
Dietary management
Galactose is ubiquitously distributed in animals and plants. It is a constituent of lactose and also certain complex lipids and proteins. Lactose, a β-galactoside in composition, occurs in the milk of mammals and is synthesised in the mammary gland [64].
The immediate and rigorous exclusion of lactose/galactose from the diet is critical in the newly diagnosed infant and should lead to amelioration of symptoms. In the long term, many complications develop despite dietary restriction. There is controversy concerning the optimal strictness and duration of dietary galactose exclusion. In particular, it is unclear how much exogenous galactose patients with classical galactosaemia tolerate and whether galactose tolerance may increase with age.
Acceptable galactose intake on a low galactose diet
There is no consensus on the degree of galactose restriction required for patients with galactosaemia and, unless taking an elemental feed, it would be difficult to have a completely galactose free diet. It has been suggested that infants may tolerate 50 mg galactose/day; toddlers 150 mg/day; children 200 mg/day; adolescents 250 mg/day; and 300 mg/day in adulthood [65]. On a strict lactose free diet (but including five portions of fruit and vegetables daily) galactose intake is calculated at 2–3 mg/kg/day in children aged between 3 and 15 years.
Sources of galactose
Although it is universally agreed that lactose must be avoided, practice regarding restriction of other dietary galactose sources varies widely throughout the world [14].
Lactose
In the UK a lactose free diet only is advocated. Lactose is cleaved into its two components, glucose and galactose, when digested and it is the main dietary source of galactose. Cow’s milk contains 4.5–5.5% lactose, i.e. about 23 g of galactose per litre. All milk, milk products and manufactured foods containing milk need to be avoided. Lactose and galactose in milk based foods are shown in Table 18.3 and Table 18.4. Constituents of milk are also likely to be found in foods such as biscuits, some sweets and tinned and processed meats (Table 18.5). Many spreads (including reduced fat) and mayonnaise include milk components to improve their flavour. The lactose content of whey derivatives such as whey powder is high, comprising 70% of total solids. In contrast, the lactose content of casein is low, with the Casein and Caseinates Regulations [66] stating the maximum anhydrous lactose content of casein should not exceed 1% by weight [67]. Butter oil and ghee contain only minimal lactose, but the recommendation is to avoid these.
Table 18.3 Lactose and galactose content of foods [67]
| Food | Lactose content (g/100 mL or g/100 g) | Galactose content (g/100 mL or g/100 g) |
| Milk, skimmed | 4.4 | 2.2 |
| Milk, whole | 4.5 | 2.3 |
| Cream, single | 2.2 | 1.1 |
| Yoghurt, low fat, fruit | 4.4 | 2.2 |
| Butter | 0.6 | 0.3 |
| Chocolate, milk | 10.1 | 5.1 |
| Ice cream, dairy | 5.2 | 2.6 |
| Lactofree milk (Arla)* | 0.02 g | 1.65 |
* Data analysed by UK Galactosaemia Support Group.
Table 18.4 Milk, milk products and milk derivatives
| Milk and milk products |
| Cow’s milk, goat’s milk, sheep’s milk |
| Cheese, cream, butter |
| Ice cream, yoghurt, fromage frais, crème fraiche |
| Chocolate |
| Milk derivatives |
| Skimmed milk powder, milk solids, milk protein, non fat milk solids, separate milk solids |
| Whey, hydrolysed whey protein, margarine or shortening containing whey, whey syrup sweetener, casein, hydrolysed casein, sodium caseinate, calcium caseinate |
| Lactose |
| Buttermilk, butterfat, butter oil, milk fat, animal fat (may be butter), ghee, artificial cream |
| Cheese powder |
| Lactose as a filler may be used in |
| Flavourings |
| Table top or tablet artificial sweeteners |
| Medicines |
Table 18.5 Lactose free diet
| Lactose free foods are listed. Manufactured foods which contain or could contain milk or milk derivatives are shown in italics. Food labels should always be checked as constituents change. |
| Milk, milk products and milk derivatives |
| These should all be avoided (Table 18.4) |
| Soya milk and soya products |
| Infant soya formula |
| Liquid soya milk – calcium enriched (not before 1 year of age) |
| Soya cheese, soya yoghurt, soya desserts |
| Fats and oils |
| Milk free margarine |
| Many margarines and low fat spreads contain milk |
| Vegetable oils |
| Lard, dripping, suet |
| Meat and fish |
| Meat, poultry, fish, shellfish (fresh or frozen) |
| Ham and bacon (lactose may occasionally be used as a flavour enhancer – see ingredient label) |
| Fish fingers |
| Quorn |
| Tofu |
| Many meat or fish products such as sausages, burgers, pies, breaded or battered foods or in sauce may not be suitable |
| Eggs |
| Cereal, flour, pasta |
| All grains; wheat, oats, corn, rice, barley, maize, sago, rye, tapioca |
| Pasta, spaghetti, macaroni, dried noodles, couscous |
| Tinned pasta such as spaghetti hoops may contain cheese |
| Flour: plain, self-raising; cornflour, rice flour, soya flour |
| Custard power, semolina |
| Carob |
| Breakfast cereals |
| Most are suitable, e.g. Weetabix, cornflakes, Rice Krispies |
| A few cereals may contain chocolate derivatives or milk |
| Bread and yeast products |
| Most bread is suitable |
| Milk bread and nan bread contain milk – avoid |
| Pitta, chapatti |
| Muffins, crumpets, teacakes may not be suitable |
| Cakes, biscuits, crackers |
| Many cakes, biscuits and crackers contain milk |
| Desserts |
| Sorbet, jelly, soya desserts, soya ice cream, soya yoghurt |
| Homemade soya milk custard or rice pudding |
| Most desserts contain milk in some form |
| Fruit |
| All fresh, frozen, tinned or dried fruit |
| Vegetables |
| All fresh, frozen, tinned or dried vegetables |
| Most dried and tinned pulses, e.g. red kidney beans, chick peas, lentils |
| Baked beans and ready made vegetable dishes such as coleslaw, potato salad may contain milk |
| Savoury snacks |
| Plain crisps, poppodom |
| Nuts, peanut butter |
| Flavoured crisps may not be suitable because they contain cheese or lactose as a filler in the flavouring |
| Dry roasted nuts and popcorn may contain milk/butter |
| Seasonings, gravies |
| Pepper, salt, pure spices and herbs, mustard |
| Marmite, Bovril, Bisto |
| Gravy granules and stock cubes may contain milk |
| Soups |
| Tinned, packet, carton soups may contain lactose |
| Sugar, sweet spreads |
| Sugar, glucose, fructose |
| Pure artificial sweeteners |
| Powdered and tablet artificial sweeteners may contain lactose |
| Jam, syrup, honey, marmalade, lemon curd |
| Confectionery |
| Boiled sweets, most mints, marshmallow, plain fruit lollies, chewing gum |
| Milk chocolate, most plain chocolate, butterscotch and fudge, toffee contain milk |
| Plain or carob chocolate may contain milk |
| Drinks |
| Soya milk |
| Milk shake syrup or powders |
| Fizzy drinks, squash, fruit juice |
| Cocoa, tea, coffee |
| Drinking chocolate may contain milk |
| Instant milk drinks and malted milk drinks – avoid |
| Miscellaneous – used in baking |
| Baking powder, yeast, gelatine, marzipan |
| Flavourings |
| Lactose may be used as a ‘carrier’ for flavourings particularly in crisps and similar snack foods. In sweets lactose is rarely used for this purpose except in some dairy flavours |
Lactose powder is added to a diverse range of food products including bakery goods, confectionery, dry mixes, dried vegetables and crisps. It is often added to prepared foods to prevent caking or as a coating. It may be used in bakery goods for enhancing browning and, as it has one-sixth the sweetness of sucrose, it is added instead of sucrose to reduce the sweetness. It is commonly used as a filler and flowing agent in seasoning mixes for foods such as instant pot noodles. It may act as a carrier for flavours and seasonings and may be added to ham, bacon and salami. It may also be added to some artificial table top or tablet sweeteners.
Non milk derivatives that may be added to food products are shown in Table 18.6. These do not need to be avoided with the exception of lactitol, a polyol made from galactose. Some residual galactose is left after its fermentation in the gut so it is unsuitable in galactosaemia.
Table 18.6 Non milk derivatives
| Lactic acid E270, sodium lactate E325, potassium lactate E325, calcium lactate E327 |
| Lactitol, lactalbumin, lactoglobulin, lycasin, stearoyl lactylates, glucona-delta-lactone, monosodium glutamate, cocoa butter, non dairy cream |
Lactitol does not contain lactose but may contain some galactose and should be avoided.
Galactosides
The α-galactosides are part of the oligosaccharides (raffinose, stachyose and verbacose) and are a potential source of galactose. They are found in foods such as peas, beans, lentils, cocoa, nuts, wheat, oat flour and vegetables [68] (Table 18.7).
Table 18.7 Dietary sources of galactosides and nucleoproteins
| Galactosides | Peas, beans, lentils, legumes, chick peas, dhals, grams, spinach |
| Texturised vegetable protein | |
| Soya (other than soya protein isolates), soya beans, soya flour Cocoa, chocolate | |
| Nuts | |
| Nucleoproteins | Offal – liver, kidney, brain, sweetbreads, heart |
| Eggs |
Studies investigating the effects of galactosides in galactosaemia are rare, include limited numbers of subjects, are short term and inconclusive. The α-galactosidase linkage in oligosaccharides is not hydrolysable by the human small intestinal mucosa in vitro or in vivo [69]. Instead, the galactosides are rapidly degraded and fermented by the caecal microflora to produce volatile short chain fatty acids. Galactoside avoidance is not advocated in galactosaemia in the UK.
Galactose storage organs
Although meats do not contain significant amounts of carbohydrates, small amounts may be present in free form and bound to proteins as glycoproteins and galactolipids. Cell lipids and proteins continuously undergo degradation, releasing glycerol, fatty acids, amino acids and their carbohydrate components, including galactose [70]. Offal is a source of galactocerebrosides and gangliosides [3]. These are not avoided in galactosaemia in the UK.
Free and bound galactose
Free galactose has been reported in many fruits, vegetables and legumes [71] (Table 18.8). Free galactose content may vary from <0.1 mg/100 g to 40 mg/100 g [72, 73]. In selected plant materials, it ranges from 2 ± 0.1 mg/100 g in red potato to 39.7 mg/100 g in red pepper. Only kiwi fruit and red pepper have galactose contents >20 mg/100 g. Different seasons, variety and storage affect the free galactose content [73]. Generally fruits and vegetables provide nutritional benefit yet induce only a small increase in daily galactose intake (30–54 mg galactose/day) and their intake is not associated with poorer outcome in galactosaemia. In one study, one patient was given a synthetic diet containing <8 mg/day galactose and then challenged with 200 mg/day galactose for 3 weeks. This had little effect on concentrations of metabolic markers [74].
Table 18.8 Free galactose in fruit and vegetables (mg/100 g)
| Food | UK* | USA† |
| Tomato | 10 | 23 |
| Grape | <10 | 2.9 |
| Cucumber | 20 | 4.0 |
| Banana | 10 | 9.2 |
| Water melon | 14.7 | |
| Kiwi fruit | 10 |
* Unpublished. Data analysed by UK Galactosaemia Support Group.
† [40, 41].
Bound galactose is also present in many plant foods as β-1-4-linked galactosyl residues in chloroplast membranes of green plant tissue. Gums and fibres (carrageenan, locust bean gum, tragacanth gum) are common sources of bound galactose. Whilst mice with galactosaemia are able to hydrolyse bound galactose in mouse chow (the hydrolysis of the bound galactose resulting in excretion of significant amounts of galactose and its metabolites in their urine [75]), in humans there is no evidence that excluding bound galactose is beneficial.
Overall, there are few data to suggest that avoiding galactose from any plant and non milk animal source is helpful. In the UK since 1983, only milk and milk products have been avoided [76] and long term outcome is not dissimilar to other countries where there are more complex and restrictive dietary management guidelines [14].
Practical management of a lactose free diet
Milk substitutes
Breast feeding and cow’s milk based infant formulas are contraindicated because they contain lactose. It is important that infants and young children are given a suitable nutritionally adequate formula. Factors influencing the choice of milk substitute include its nutritional composition, the child’s clinical and nutritional status, safety and palatability of the product, ease of preparation, cost and availability.
Soya infant formula
Soya infant formula is commonly the first choice for centres treating babies with galactosaemia [14] and is supported by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [77] and the UK Galactosaemia Medical Advisory Panel. This is mainly because there are few other safe options to use in infancy. Nutritionally complete soya infant formulas, e.g. InfaSoy, Wysoy, are lactose free and oligosaccharides are removed during manufacture. Estimates suggest that they may contain 1–2 mg galactose per 100 mL [78].
One potential disadvantage of soya infant formula is that it is a rich source of isoflavones, particularly genistein and daidzein. These compounds are structurally similar to the mammalian oestrogen hormone but are much less potent (0.1%–1% of the oestrogenic potential of 17-β-oestradiol [46]). They can bind to oestrogen receptors and act as anti-oestrogenic agents by blocking oestrogen and this was identified as a concern by the UK Food Standards Agency [79] and Department of Health [80]. There is no evidence that the use of soya infant formula is a factor in the fertility problems seen in galactosaemia.
Low lactose and protein hydrolysate formula
Other alternative infant formulas include low lactose formulas and casein hydrolysate formulas (Table 18.9). Medium chain triglyceride (MCT) based casein hydrolysate infant formula, such as Pregestimil Lipil, may be advantageous when there is significant liver disease and should be administered until this complication has resolved [3].
Table 18.9 Low lactose, protein hydrolysate and amino acid formulas for infants with galactosaemia
| Infant formula | Protein source | Lactose content (per 100 mL) |
| Low lactose | ||
| Enfamil O-Lac (Mead Johnson) | Milk protein (isolate) | <7 mg |
| Galactomin 17 (SHS/Nutricia) | Caseinate | <10 mg |
| SMA LF (SMA Nutrition) | 60% whey, 40% casein | <10 mg |
| Casein hydrolysate | ||
| Nutramigen Lipil 1 and 2 (Mead Johnson) | Enzymatically hydrolysed casein | <5 mg |
| Pregestimil Lipil (Mead Johnson) | Enzymatically hydrolysed casein | <5 mg |
| Whey hydrolysate | ||
| Pepti-Junior (Cow & Gate) | Hydrolysed whey protein | 0.1 g |
| Meat and soya hydrolysate | ||
| Pepdite (SHS/Nutricia) | Hydrolysed pork and soya amino acids | Nil |
| Amino acid formula | ||
| Neocate LCP (SHS/Nutricia) | L-amino acids | Nil |
| Nutramigen AA (Mead Johnson) | L-amino acids | Nil |
| Neocate Nutra (SHS/Nutricia) This is not a complete formula. Can be used from 6 months (as a paste) as part of a nutritionally adequate diet | L-amino acids | Nil |
However, all low lactose and protein hydrolysate formulas based on cow’s milk protein will undoubtedly contain some residual lactose and the amounts vary. Manufacturers of these formulas declare their lactose content at <10 mg/100 kcal (420 kJ) as defined by the European Food Safety Authority (EFSA) [81]. A 5 kg infant taking 200 mL/kg of formula would have <10 mg/kg/day (or 50 mg/day) of galactose from this source. However, it is estimated that endogenous galactose production is likely to be at its highest in infancy (calculated at 41 mg/kg body weight/day) [59], so it is probably better to use a formula which contains no source of lactose.
Whey hydrolysate formulas are likely to contain a higher concentration of residual lactose and Pepti-Junior, for example, is likely to contain 100 mg/100 mL of lactose (personal communication) and are unsuitable. Enteral feeds for older children based on whey hydrolysates are unsuitable.
Any formula based on hydrolysed meat and soya should be free of lactose and suitable to use.
Amino acid based formula
This alternative is a suitable lactose free formula and is used by some centres as the first choice in babies with galactosaemia [14]. There have been at least three case reports of infants with galactosaemia given elemental (amino acid based) infant formulas successfully [82, 83] and they may offer an advantage as they are more likely than soya infant formulas to be fortified with novel nutrients such as long chain polyunsaturated fatty acids. The main challenge when using elemental formulas is to ensure infants establish a taste for soya based foods during the weaning period.
Vegetable based milks for children over 1 year of age
A number of commercial nutritionally enriched plant, cereal and nut based drinks (e.g. soya, oat, rice, hazelnut and almond) can be purchased in supermarkets, specialist shops or on the internet. Most of them contain approximately 120 mg/100 mL calcium and 0.8 µg/100 mL vitamin D. There are also nutritionally enriched follow on soya milks designed for children >1 year with added calcium, iron, vitamin D, some B vitamins and an energy profile similar to cow’s milk. Calcium absorption from soya drinks is similar to that from cow’s milk. Soya beverages made from soya protein isolate contain less free galactose (1.3 ± 0.2 mg/100 g) compared with whole soya beans (4.8–5.3 ± 1.7 mg/100 g) [73]. Rice milk contains traces of inorganic arsenic and it is recommended by the UK Food Standards Agency that children aged between 1 and 4½ years should avoid rice milk.
Unsafe milks
Since lactose is the carbohydrate source in animal milks, e.g. goat’s and sheep’s milks, these are unsuitable. A low lactose dairy drink, Lactofree (Arla), where about half the lactose is removed by filtration and the remainder is removed enzymatically, is available internationally. However, recent analysis has demonstrated that it contains a significant quantity of galactose, rendering it unsuitable for galactosaemia (Table 18.3) [84]. Lactase enzyme drops, which reduce the lactose content of cow’s milk, are also unsuitable.
Cheeses
Hard mature cheeses
A small number of hard mature cheeses are allowed. Most cheese types have high levels of casein (which contains no more than 1% lactose) and low levels of whey (which contains 70% lactose). The lactose content is reduced during the cheese making process: by removal of whey by drainage (during this stage approximately 98% will be removed); type of starter culture and coagulating enzyme can have an effect as can the acid produced – all influence the properties of the curd and the degree of whey expulsion. For harder cheeses the curd is cut into small cubes, which allows fluid to drain from the individual pieces of curd. Any residual lactose in cheese curd is metabolisd during its ripening [85] and, generally, the longer the cheese has matured the lower its lactose content.
In the UK, the following cheeses are allowed: Emmental, Gruyere, Jarlsberg, Parmigiano Reggiano and Grana Padano Italian Parmesans with an EU PDO (Protected Designation of Origin) seal on the label; also West Country Farmhouse mature Cheddar cheese with an EU PDO seal on the label. All of these cheeses have been extensively tested for their lactose and galactose content [86] and have been shown to contain minimal lactose and galactose on repeated analysis. Information regarding suitable West Country Farmhouse mature Cheddar cheese is available from www.farmhousecheesemakers.com. A PDO seal only guarantees that a food genuinely originates from a region and is produced to a specified quality; it does not indicate that it contains minimal lactose. Many food stuffs carry the PDO seal, e.g. other types of cheese, sausages, hams and regional breads, and these are not necessarily lactose free. However, the PDO seal should always be on the label of any West Country Farmhouse Cheddar cheese or Parmigiano Reggiano and Grana Padano Italian Parmesans to indicate their suitability.
All other cheeses are not permitted. Specific analysis has been commissioned by the UK Galactosaemia Medical Advisory Panel to test Gouda, Edam and some soft and processed cheeses, but all contain lactose and galactose; Mini Emmental Baby Bel contains a mean of 6–7 mg/100 g lactose and so is also unsuitable. The latter cheese is only fermented for 196 days and is usually on the retail market by 32 weeks of age [87].
Enzyme treated cheese products
A Lactofree Cheddar type of cheese has been produced from Lactofree (Arla) milk. Initial findings suggest this is low in lactose and galactose [84], but as there has been only limited analysis it is considered unsuitable in the galactosaemia diet.
Inclusion of cheese in the diet
There are different guidelines in different countries on the inclusion of cheese in the diet for galactosaemia. A survey of European practices [88] found that hard cheese such as Emmental and Gruyere were allowed in 63% of countries. Gouda was allowed in some countries but not others. In Australia and New Zealand some mature Cheddar cheese made by traditional methods are allowed [89]. In the USA some clinics allow mature hard cheese, e.g. Emmental, Gruyere, Tilsider [78].
Acceptability of low lactose/low galactose cheese
For many patients and carers the allowance of low lactose, but milk derived, cheese is a difficult concept to comprehend. However, a recent survey conducted by the UK Galactosaemia Support Group indicated there was a wide acceptance with 76% of patients eating one or more suitable types of cheese. The most preferred of the low lactose/low galactose cheeses were West Country Farmhouse Cheddar (70%), Emmental (31%), Italian Parmesan (24%), Gruyere (18%) and Jarlsberg (14%) [90].
Manufactured foods
Interpreting food labels
European ingredient listing rules [91–93] applied across the EU give clear guidance about food labelling of prepackaged foods that contain intentional milk, milk derivatives or lactose, which have to be clearly identified. Milk or lactose in carry-over additives or flavourings, and any other substances used as processing aids are also acknowledged. This only applies to prepackaged foods, e.g. canned and packet foods and alcoholic drinks, so foods sold loose (i.e. unpackaged), foods served in restaurants and certain fancy confectionery products are exempt.
However, the UK Foods Standards Agency (FSA) [94] has introduced The Provision of Allergen Information for Non Prepackaged foods – Voluntary Best Practice Guidance, targeted at food producers. It is requested that food suppliers communicate honestly with customers about the contents of any unpackaged foods that are sold; and that all employees receive training about food allegens (including milk), have up to date knowledge about the ingredient content of foods sold, consider cross-contamination and have an agreed practice for dealing with allergy information. The FSA has also launched a Chef Allergen card. The customer’s food allergy requirements are completed on a credit card which is handed to a chef who will then be able to decide if they are able to produce a lactose free meal for the individual. At the end of 2014, new UK legislation will be introduced that will require businesses to provide allergy information on food sold unpackaged in catering outlets, deli-counters, bakeries and sandwich bars.
Cross-contamination of manufactured foods with milk
Both milk free and milk containing foods may be manufactured in the same plant or even using the same machinery. Cross-contamination with milk is always possible and such foods may be voluntarily labelled may contain milk or milk products. It is likely that the quantity of milk that such a product would contain if cross-contaminated would be minute. The UK Galactosaemia Support Group Medical Advisory Panel permits foods that only carry a risk of cross-contamination with milk.
Vitamin and mineral supplementation
Many nutrients are involved in optimum bone metabolism, particularly calcium, phosphate and vitamin D. Vitamin K also has an important role to play, acting as a cofactor in the post translational carboxylation of osteocalcin which has a regulatory role in the mineralisation and remodelling of bone [40]. Some recommend routine vitamin K supplementation in addition to calcium and vitamin D in galactosaemia [95]. Maintenance of adequate cellular zinc concentrations is also important for growth and bone metabolism; bone specific alkaline phosphatase and osteocalcin are zinc dependent [37].
It is important to ensure that patients meet the reference nutrient intake (RNI) for all bone related nutrients. Calcium intake is commonly low in galactosaemia [39, 96, 97]. The UK RNI for calcium varies between 325 mg and 1000 mg daily in children and teenagers [98]. Wherever possible, calcium requirements should be achieved by eating foods. Soya infant formula normally provides adequate calcium in the first 1–2 years if sufficient amounts are taken, but it may be necessary to change to a calcium enriched soya drink (preferably supplemented with vitamin D), encourage low lactose cheese, soya yoghurts, sardines, mackerel, sesame seeds, lentils and milk free fortified bread to ensure adequate calcium intake (Table 18.10). Dietary assessment should be performed annually to ensure sufficiency.
Table 18.10 Food sources of calcium for galactosaemia [67]
| Calcium (mg) | Vitamin D (µg) | |
| per 100 mL/100 g | ||
| Calcium/vitamin D enriched soya drinks | 100–140 | 0.8 |
| Soya dairy free yoghurts | 120 | Variable |
| Low lactose hard cheese | 730 | 0.3 |
| Whitebait | 860 | Not available |
| Sardines in oil/brine | 500 | 5 |
| Pilchards | 250 | 14 |
| Sesame seeds | 670 | 0 |
| Red kidney beans | 100 | 0 |
| Bread | 170 | 0 |
| Spinach | 170 | 0 |
| Apricots | 73 | 0 |
If calcium intake is low, it is necessary to give a calcium supplement which may be challenging mainly because of the size, type of presentation (mainly tablets rather than liquid) and nutritional composition of the calcium supplements available for children. Additional vitamin D supplementation will aid calcium absorption, e.g. Healthy Start vitamin drops (p. 20) . Alternatively, there are a number of prescribable calcium supplements with added vitamin D (Table 18.11). The latter are mainly chewable or dissolvable in water. They are bulky, unpopular with children and adherence is an issue. There are no suitable liquid calcium supplements listed in the British National Formulary (BNF) for children. If dietary intake is poor, it may be necessary to give a more comprehensive vitamin and mineral supplement in the form of Paediatric Seravit or Fruitivits (suitable from 3 years of age).
Table 18.11 Suitable calcium supplements for galactosaemia
| Presentation | Available calcium (mg) | Vitamin D (µg) | |
| Adcal-D3 (ProStrakan) Calcium carbonate Chewable tablet/effervescent tablets | Tablet | 600 | 10 |
| Cacit-D3 (Proctor and Gamble Pharm) Calcium carbonate Granules | Sachet | 500 | 11 |
| Calceos (Galem) Calcium carbonate Chewable tablet | Tablet | 500 | 10 |
| Calcichew D3 (Shire) Calcium carbonate Chewable tablet | Tablet | 500 | 5 |
| Calcichew D3 Forte (Shire) Calcium carbonate Chewable tablet | Tablet | 500 | 10 |
| Natecal D3 (Chiesi) Calcium carbonate Chewable tablet | Tablet | 600 | 10 |
| Sandocal+D 600 (Novartis) Calcium lactate gluconate, calcium carbonate Effervescent tablet | Tablet | 600 | 10 |
Calcium Sandoz Liquid (Alliance) is based on calcium lactobionate. It is contraindicated in galactosaemia because β-galactosidase in human intestinal mucosa hydrolyses lactobionate freeing galactose (Calcium Sandoz Liquid medical information [114]). The amount of galactose released is unknown.
There is a suggestion that calcium supplementation may increase the risk of myocardial infarction [99, 100]. This may be related to an acute increase in serum calcium observed after calcium supplements but not dietary calcium [100]. An increase in serum calcium levels has been associated with vascular calcification [101]. Ideally calcium supplements should be co-administered with vitamin D and given as more than one daily dose.
Dietary education
It is important for families to be educated about lactose free food choices and the importance of healthy eating and appropriate nutrition (and exercise) for healthy bones. They also need help with choosing vitamin/mineral supplements. Interpretation of food labelling, suitability of unpackaged food items, lactose free cooking and choice of appropriate school meals are important. Dietary education of children is essential, particularly with the interpretation of food labels to enable them to take control of their own diet and make independent food choices. This will help foster curiosity and a healthy attitude towards their diet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree