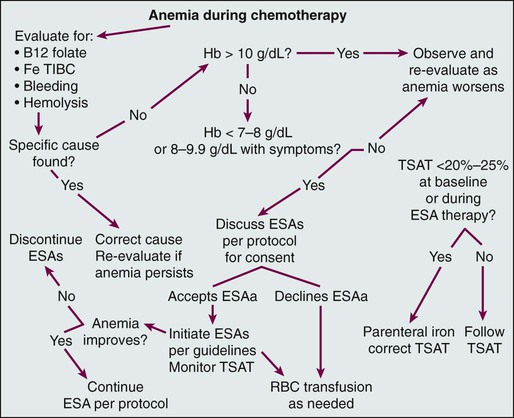
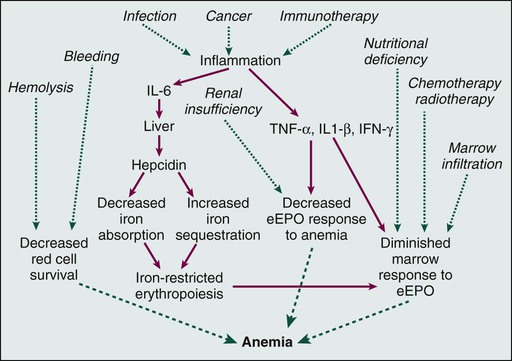
• Anemia is very common in patients with cancer and is multifactorial. • The anemia of persons with chronic disease is associated with decreased absorption of oral iron and decreased ability to access storage iron pools. • Iron-restricted erythropoiesis may limit the efficacy of erythropoietic agents for the treatment of anemia in these patients and can be overcome with parenteral iron. • Treatment of anemia in patients with cancer reduces transfusions and symptoms of anemia. • Erythropoietic agent therapy is associated with an increased risk of venous thromboembolism. • Neutropenia in patients with cancer is usually due to treatment. • Myeloid growth factors can be used to reduce infection risk in patients for whom the risk is significant, and use of these growth factors is preferable to delaying or reducing the dose of chemotherapy when prolonging life is the intent. • New agents for the stimulation of platelet production are in clinical development. • Biosimilar preparations for commonly utilized hematopoietic growth factors will be appearing on the U.S. market. It will be important for oncologists to participate responsibly in postmarketing surveillance programs. Disorders of blood cell production, which usually are manifested as anemia, leukopenia, or thrombocytopenia, are both very common and enormously important in the clinical practice of oncology. Under ordinary conditions in the healthy adult, blood cell production is extraordinarily prolific, with daily outputs in the range of 2 × 1011 erythrocytes,1 5 × 1010 neutrophils,2 and 2.5 × 1011 platelets,3 along with substantial numbers of lymphocytes, macrophages, antigen-processing cells, eosinophils, and basophils. With more than 5 million blood cells produced every second even under ordinary conditions, the mitotic yield of normal bone marrow is far greater than that of almost any malignancy, where the production of a similar number of new cells would result in a daily increase of tumor cell burden of more than 0.25 kg per day. Therefore it is not surprising that the most common unintended consequences of cancer treatments with antimitotic mechanisms of action are clinically important degrees of anemia, neutropenia, or thrombocytopenia. The rate of blood cell production is both tightly regulated and highly variable. Under conditions of either increased destruction of cells, such as bleeding, hemolysis, or immune destruction of platelets, or demand for an increased numbers of cells, such as infection, production rates of appropriate cells increase several fold. The regulation of this dynamic system is complex4 but for practical purposes can be conceived of as involving an interaction between a pool of pluripotent hematopoietic stem cells, which are capable of both infinite self-renewal and differentiation into mature blood cells, and regulatory factors, including both a well-characterized set of glycoprotein hematopoietic growth factors and a less well-understood group of inhibitory factors. Cancer and its treatment are very often associated with profound perturbations in this system of controlling blood cell production. Understanding this biology is key to rational intervention and optimal care of patients with cancer. Anemia is common in patients with cancer5,6 and is often multifactorial, with frequent contributors including bleeding, general malnutrition, iron, folate or vitamin B12 deficiency, hemolysis, myelosuppressive chemotherapy, radiation to marrow-bearing bones, relative endogenous erythropoietin deficiency, and the anemia of chronic illness. In addition to these factors, for B-cell malignancies and solid tumors extensively involving the marrow, disruption of the normal interactions of hematopoietic progenitor cells and endothelial and stromal cells in the marrow microenvironment may play an important role. For patients with secretory multiple myeloma, renal insufficiency is a frequent and often unrecognized factor in the anemia. Finally, for patients with myelodysplasia and myeloid malignancies, the hematopoietic stem cells themselves are reduced in number and/or dysfunctional. It has been recognized for some time that patients with chronic inflammatory illnesses, including cancer, frequently have a diminished endogenous erythropoietin (EPO) response to anemia.7 More recently, it has been recognized that inflammatory conditions, including cancer, are frequently associated with increased production of hepcidin, an iron-regulatory peptide, by the liver.8–11 Hepcidin binds to and inactivates the iron transporter ferroportin, impairing both absorption of dietary iron and access to storage iron pools.12,13 These observations suggest that iron-restricted erythropoiesis may occur commonly in patients with cancer, despite the presence of what are believed to be adequate iron stores, because access to storage and dietary iron is impaired. Our current understanding of the biology of anemia in patients with cancer is shown in Figure 34-1. Anemia has potential consequences for patients with cancer. Anemia can result in diminished oxygen delivery to normal tissues and result in symptoms such as fatigue and decreased exercise tolerance. Elderly patients or patients with comorbidities that are common in association with cancer, such as chronic lung disease, ischemic heart disease, or pleural effusions, are particularly vulnerable to these symptoms. Red blood cell transfusions have become much safer with advances in transfusion medicine, but passage of infections, transfusion-related acute lung injury, hemolytic transfusion reactions, and iron overload are still a risk. When these events occur, they can be regarded as additional adverse effects of anemia for patients with cancer. Anemia can also result in diminished oxygen delivery to tumor cells and may thereby enhance the degree of hypoxia in the tumor microenvironment.14–38 A long-standing concern is that tumor cell hypoxia may result in the development of more aggressive and/or apoptosis-resistant tumor phenotypes17,39–51 and reduce the efficacy of radiotherapy52–59 or chemotherapy.23,60–62 Across tumor types, anemia is a negative prognostic factor,63 although it has not been shown that anemia is a cause of reduced response or survival as opposed to a marker of a more ill patient or a more advanced malignancy. Despite the interest and the provocative data previously summarized, it has never been conclusively demonstrated that any tumor cell hypoxia that occurs as a result of anemia in clinical oncology practice results in an adverse outcome for the cancer or affects the efficacy of treatment. It is surprising how frequently the anemia observed in patients with cancer is due, at least in part, to reversible factors, such as iron loss through bleeding or excessive phlebotomy or previously unsuspected vitamin B12 deficiency. In one study, 5% of patients undergoing chemotherapy for cancer who were being considered for inclusion in an erythropoiesis-stimulating agent (ESA) and parenteral iron clinical trial were found upon screening evaluation to have below-normal serum vitamin B12 concentrations.64 Treatable causes can and should be addressed; historically, the only available treatments for the remaining patients with cancer and anemia were red blood cell transfusions and successful treatment of the underlying malignancy. Because of the well-known risks associated with transfusions and the need to conserve a limited blood supply, specific anemia treatment was limited to patients with profound degrees of anemia (i.e., hemoglobin [Hb] levels 8 g/dL or lower) or serious cardiovascular symptoms, such as chest pain or dyspnea at rest. The key regulator of red blood cell production is the glycoprotein hormone EPO. The cloning, development, and introduction into clinical use of recombinant human EPO represented a watershed in anemia management. Two preparations of recombinant ESAs are currently available in the United States, epoetin alfa and the hyperglycosylated recombinant EPO darbepoetin alfa,65 which has a longer half-life. In randomized, placebo-controlled trials, both epoetin alfa66–70 and darbepoetin alfa71,72 have been shown to reduce red blood cell transfusion rates in patients with cancer receiving chemotherapy, and both agents are approved by the U.S. Food and Drug Administration (FDA) for this indication. Historically, it had been observed that even mild and moderate degrees of anemia can impair function and limit productivity in otherwise healthy adults.73 When ESAs became available and were applied to the treatment of patients with renal failure, it was shown that quality of life and productivity improved when Hb levels increased and that this relationship continued to hold even at Hb levels well above the traditional transfusion threshold.74–77 Comparisons of data gathered during ESA treatment of anemic patients undergoing dialysis with data from anemic patients who had cancer suggested that the effects of anemia and the benefits of treatment in terms of improved quality of life and energy level were quite similar in the two settings.78 Analyses of the relationship between Hb level and energy, activity, and overall quality of life observed in large, uncontrolled series of patients with cancer during treatment for anemia with epoetin alfa suggested that larger incremental increases in these patient-reported outcomes were observed with Hb increases from 11 to 12 g/dL than with any other incremental 1-g increase.79 Two large surveys had demonstrated that fatigue is common and often is the dominant symptom in patients with cancer in the United States, limiting function and quality of life.80,81 When analysis of data from several trials, some of them randomized, indicated that ESA therapy for anemia is associated with improvements in fatigue in patients with cancer,69,70,82–96 a second goal of ESA therapy beyond transfusion reduction emerged: maintenance of functionality through relief of anemia-related symptoms, particularly fatigue. Not all trials have confirmed this effect on patient-reported outcomes,97 and no ESA is currently approved by the FDA for relief of fatigue or improvement in quality of life in anemic patients with cancer; transfusion reduction remains the sole label indication. Both ESAs are currently used for the treatment of anemia in patients with cancer who are undergoing chemotherapy, and randomized trials to date have failed to demonstrate that either agent is superior in terms of transfusion prevention or fatigue reduction when used at starting doses of 40,000 units per week for epoetin alfa and 200 µg every 2 weeks for darbepoetin alfa.98 It has been shown that darbepoetin alfa is also effective for the treatment of chemotherapy-associated anemia when given every 3 weeks at doses of either 300 µg99 or 500 µg.100 Every-3-week dosing on the same day as chemotherapy is as effective as asynchronous dosing.101 It also appears feasible to administer epoetin alfa on an every-3-week basis at a dose of 120,000 units for the treatment of anemia during cancer chemotherapy.102,103 No evidence has been found that higher doses of either ESP result in improved outcomes for patients with cancer, and although it was once a common practice to increase doses in hyporesponsive patients, this approach has never been studied and its benefit, if any, is unknown. It is important to bear in mind that weeks are usually required before ESA therapy increases Hb levels, and the only rapid remedy is red blood cell transfusion for acute intervention in persons who have severe anemia and ominous symptoms such as chest pain and dyspnea at rest. The relatively slow onset of ESA effects has important implications for the optimal utilization of ESAs for minimizing transfusion needs in patients with chemotherapy-induced anemia. When intervention with an ESA is withheld until the Hb level is less than 9 to 10 g/dL, some responsive patients will require transfusions for acute management of severe anemia before they respond to treatment. Several trials have prospectively addressed the issue of early versus late intervention, and taken in aggregate, the results suggest that later intervention is associated with a substantial increase in transfusion rates.104 Accordingly, before the recent safety concerns for ESAs in oncology arose (discussed later in this chapter), most guidelines suggested initiation when Hb levels fell below 11 g/dL, especially for patients predicted to be receiving multiple additional cycles of chemotherapy with rapidly falling Hb levels and/or symptomatic anemia.105 In the United States, guidelines have recently been amended because of safety concerns, and it is now recommended that ESAs be used in patients with cancer who are undergoing chemotherapy only when the Hb level is less than 10 g/dL.106,107 Risk mitigation procedures have been put in place to ensure that physicians are aware of these restrictions and that patients are adequately informed of potential risks. Europeans, although presented with the same risk/benefit data, have continued to allow for earlier initiation of ESA treatment within their guidelines.108,109 When ESAs are given to patients who have anemia related to renal failure, an increase in platelet count is sometimes observed. Although this response was initially believed to reflect an effect of EPO on megakaryocyte growth and development, it has since been shown to reflect inadequacy of iron supply to the marrow.110 In retrospect, this effect was the initial evidence of something important: ESA therapy taxes the ability of the marrow to access storage iron, and iron-restricted erythropoiesis can ensue, even in the presence of apparently adequate body iron stores.111 This phenomenon, which is thought to be due to an inability to mobilize storage iron rapidly enough to support the accelerated erythropoiesis associated with ESA treatment, has been termed “functional iron deficiency” to distinguish it from the more familiar absolute iron deficiency reflective of diminished total body iron stores. The limited quantity of oral iron that can be absorbed on a daily basis, coupled with the poor gastrointestinal tolerance of and patient compliance with oral iron ingestion, makes parenteral iron an attractive option for addressing functional or absolute iron deficiency before and during ESA therapy. Although early high-molecular-weight preparations of iron dextran were associated with infrequent but potentially life-threatening anaphylactic reactions,112 the newer low-molecular-weight dextran preparation (INFeD), sucrose or gluconate iron salts, and the carbohydrate-coated iron oxide nanoparticle preparation (ferumoxytol) are relatively safe.113–116 A summary of the available parenteral iron preparations and practical aspects of their administration is contained in E-Table 34-1.117 E-Table 34-1 Summary of Available Parenteral Iron Preparations ESP, Erythropoiesis-stimulating protein; LBW, lean body weight; TDI, infusion of the total dose. *Auerbach M, Witt D, Toler W, et al. Clinical use of the total dose intravenous infusion of iron dextran. J Lab Clin Med 1988;111:566-70; and Auerbach M, Winchester J, Wahab A, et al. A randomized trial of three iron dextran infusion methods for anemia in EPO-treated dialysis patients. Am J Kidney Dis 1998;31:81-6. †Auerbach M, Chaudhry M, Goldman H, Ballard H. Value of methylprednisolone in prevention of the arthralgia-myalgia syndrome associated with the total dose infusion of iron dextran: a double blind randomized trial. J Lab Clin Med 1998;131:257-60. For patients with renal failure who are undergoing dialysis, administration of parenteral iron has been shown to reduce the required ESA dose and to improve ESA response118 in ESA-resistant patients.119 Chronic inflammatory illnesses, including cancer, can be associated with diminished absorption of oral iron and decreased accessibility of body iron stores. When patients with this anemia of chronic illness receive ESA therapy, the increased iron demand by the erythron can result in functional iron deficiency and iron-restricted erythropoiesis. Unfortunately, all of the clinical development of ESAs for anemic patients with cancer was performed before the realization that parenteral iron was an important unanswered question, and the data about parenteral iron that have been subsequently accumulated call into serious question the conclusions of those trials regarding optimal ESA dose and schedule and efficacy. In the first randomized trial to address this issue, iron dextran, given either as a weekly fixed dose containing 100 mg of elemental iron or as a single total dose infusion to anemic patients with cancer who were undergoing chemotherapy, was associated with a significantly better response to epoetin alfa than that observed with either oral iron or no iron support.120 Similar results have been reported in several subsequent trials utilizing various ESAs and parenteral iron preparations.121–124 Taken together, these trials indicate that parenteral iron therapy will both enhance ESA response and reduce ESA requirements in the oncology setting, similar to the established role for patients undergoing dialysis. A challenge to rational iron support during ESA treatment of patients with cancer is the reliable detection of iron-restricted erythropoiesis in this patient population. The anemia of persons with a chronic illness is associated with reductions in serum iron and iron-binding capacity and with increases in serum ferritin levels, rendering transferrin saturation and ferritin determinations less reliable indicators of adequate iron delivery to the marrow or of body iron stores.125 Serum levels of soluble transferrin receptors are normal in patients with the anemia of chronic disease and increased in patients with iron deficiency anemia and therefore might be useful in distinguishing the two conditions; however, this laboratory parameter is not widely available. Moreover, soluble transferrin receptor levels are increased by ESA treatment and fluctuate during the chemotherapy cycle, which makes their future usefulness for monitoring iron supply to the marrow during ESA therapy less promising in anemic patients with cancer who are undergoing chemotherapy. Similar limitations may apply to the use of the transferrin receptor-to-ferritin ratio.126 Two additional parameters that have been proposed include the percentage of hypochromic red cells127,128 and the reticulocyte hemoglobin content.129–135 The relationship of these parameters to iron delivery to the marrow is not affected by the inflammatory milieu of chronic illness, ESP therapy, or chemotherapy, and both of these parameters are available on some blood count autoanalyzers. The data suggest that when the proportion of red blood cells with a Hb concentration of less than 28 g/dL exceeds 5%, it can be concluded that significant iron restriction of erythropoiesis has occurred during the preceding 2 weeks. The data also imply that, although the usefulness of the reticulocyte Hb test is limited in the presence of macrocytosis, when the reticulocyte Hb content is less than 29 pg, iron-restricted erythropoiesis has occurred during the preceding 2 days. It should be cautioned that because these tests have not been validated for monitoring iron-restricted erythropoiesis136 and are both validated and more broadly available for monitoring iron supply during ESA therapy for chemotherapy-associated anemia, it is prudent to consider parenteral iron therapy whenever the transferrin saturation is less than 25% to 30% or when the response to ESA therapy is inadequate. If the percentage of hypochromic red blood cells or reticulocyte Hb content is available, these values can be integrated into the evaluation. An algorithm for the management of anemia during chemotherapy for cancer is shown in Figure 34-2. ESAs are generally well tolerated, although three issues regarding their safety merit consideration on the part of the oncologist. First, shortly after the introduction of a new formulation of epoetin alfa in Europe and Canada, an increase in pure red cell aplasia (PRCA) was noted in patients with renal failure who were receiving ESP therapy.137 This complication was found to be caused by autoantibodies to epoetin, which apparently developed in response to a subtle alteration in the tertiary structure of the recombinant protein and were cross-reactive with endogenous EPO. When the problem was recognized, changes were made in the production, storage, and handling of that formulation, and the observed increase in PRCA resolved.138 This problem was not observed in patients with cancer who were treated with that ESA, presumably because the duration of therapy was insufficient. This episode raised an important issue relevant to all cloned protein therapeutics with a homolog in the human: the potential for immunogenicity. Because of extensive posttranslational modifications in cloned proteins that vary significantly with different production techniques, protein products from different manufacturers, despite identical amino acid sequences, can have very different degrees of immunogenicity. Because patents are expiring on the initial cloned human proteins, including erythropoietin and granulocyte colony-stimulating factor (G-CSF), new preparations will soon be appearing on the market and entering clinical practice. This process has already begun in Europe and will begin in the United States by 2014. Given the infrequency of autoantibody development even when a given preparation has significantly greater immunogenicity, it is likely that, if an increase in autoantibodies occurs, it will first be observed during postmarketing surveillance. It will be very important for clinicians to be observant for signs of autoantibody events and be aware of which preparation(s) of ESAs and other cloned human proteins their patients have been receiving. Recently, peginesatide, an erythropoietin receptor (EPO-R) agonist with no sequence homology with ESAs or endogenous human EPO, was introduced into the market for the treatment of anemia in patients undergoing dialysis.139 This agent is effective in the treatment of PRCA associated with ESA use, and it can be utilized as a rescue therapy in the rare event that PRCA occurs. The second established safety issue with ESAs is venous thrombosis. Metaanalyses of randomized, placebo-controlled trials of ESAs administered to patients with cancer have demonstrated an increase in the incidence of venous thrombotic events in patients receiving these agents.140 The overall relative risk of thrombosis associated with ESAs is 1.4 to 1.7; rather than the incremental risk being spread evenly over all patient groups, it appears to be greater in patients with gynecologic malignancies (although not all studies have confirmed this finding141), patients receiving combined radiotherapy and chemotherapy treatment regimens, and patients who also receive red blood cell transfusions.141–144 The increase in thrombosis is not a cancer-specific observation; it is observed in patients with renal failure and critically ill patients145 who receive ESAs. The mechanism(s) by which ESA therapy increases thrombosis risk is unknown; significant correlations of thrombotic events with highest Hb level, rate of Hb rise, or ESA dose have not been observed with sufficient consistency to permit a conclusion that the increased risk is due, in whole or in part, to altered blood rheology, although this conclusion remains a possibility.148–148 The increase in diastolic blood pressure that can occur with the initiation of ESA treatment before Hb levels rise suggests the possibility of a direct effect on vasculature, and some biochemical evidence has been found of endothelial cell and platelet activation149,150 and endothelial cell mobilization151 during ESA treatment in humans. This effect of ESAs on vascular endothelial activation may be counteracted by endothelial nitrous oxide production, at least in animal models.152 In vitro evidence indicates that ESAs may synergize with thrombopoietin (TPO) in inducing platelet activation153 and/or that platelets may be activated through interaction with young red blood cells.154 Further studies are needed, both to elucidate the mechanism(s) of ESA-induced thrombosis and to establish rational approaches to prediction and prevention. At this time, no evidence has been found that venous thrombosis associated with ESAs can be reduced by low-dose warfarin155 or by fish oil,156 although full antithrombotic prophylaxis may reduce or eliminate the incremental risk.157 The most controversial issue with respect to the safety of ESAs in patients with cancer has been their potential effects on tumor progression and survival. Prior to 2005, it was hoped that amelioration of anemia would enhance tumor oxygenation and improve cancer outcomes.158,159 The preclinical data had suggested that this might be the case, and early metaanalyses showed a trend suggesting a possible positive impact of ESA therapy on the survival of patients with cancer.160,161 Subsequently, in two randomized trials of ESAs used to prevent rather than to treat anemia in patients with breast cancer who were receiving chemotherapy162,163 or in patients with head and neck cancer who were undergoing radiation therapy,164,165 an increase in mortality or a decrease in the time to tumor progression was observed in patients treated with ESA. Although both trials had serious methodologic issues,166 these results correctly raised concerns regarding the safety of ESAs in patients with cancer and highlighted the need for appropriately designed and executed randomized trials with tumor progression and overall survival as primary end points, carried out in patients with established anemia related to ongoing cancer chemotherapy. Those trials are now in progress, one for patients with lung cancer, and the other for patients with breast cancer; only the results of those trials can settle this issue definitively. Shortly after the results of the two aforementioned trials were reported, investigators reported similar findings for tumor progression or survival in some additional trials that were often not designed with tumor progression or survival associated with ESA therapy for anemia as the primary end point.167 These trials included patients with B-cell malignancy,72 patients with cervical cancer who were undergoing radiochemotherapy,168 patients with non–small cell lung cancer receiving no chemotherapy or second-line chemotherapy,169 nonanemic patients with head and neck cancers receiving radiation therapy (the DAHANCA-10 trial, not yet published170), and patients receiving neoadjuvant chemotherapy for breast cancer.171,172 Also during this time frame, trials reporting conflicting observations have been reported for patients with B-cell malignancies including Hodgkin disease173 and non-Hodgkin lymphoma (the GELA trial, not yet published174), cervical cancer,175 non–small cell lung cancer,71,105,176 small cell lung cancer,179–179 head and neck cancers,180 and patients with early breast cancer (German WSG ARA trial, not yet published181) and metastatic breast cancer.96,182 Metaanalyses of randomized, controlled trials of ESA treatment for established anemia during cancer chemotherapy have not shown an increase in tumor progression or a decrease in overall survival in anemic patients treated with ESAs.140,146,161,176,183–186 A more recent metaanalysis focusing on on-study mortality has found that, for that end point, ESA therapy, even in the chemotherapy setting, is associated with increased mortality.187,188 However, because the vast majority of randomized trials have observed little or no on-study mortality, this approach has the effect of disproportionately weighting the contribution to the conclusion of only two trials that did observe on-study mortality, which limits the rationale and value of a metaanalysis.189,190 It is likely that we have gone as far as we can, and probably farther than we should, with metaanalyses for this issue.191 Settlement of this controversy requires randomized trials that are designed to study a single tumor type and anticancer treatment and are stratified for baseline factors known to predict tumor progression and survival, with sufficient power, protocol adherence, and follow-up, with ESAs used to treat anemia. Two such trials are in progress. If ESAs do affect the progression of cancers, the mechanism for this effect is not known, which complicates the development of rational strategies for optimizing anemia management in persons with cancer. Three major mechanisms have been postulated to explain an effect of ESAs on tumor outcomes.192 Some investigators have postulated that ESAs have a direct effect on tumor cells by acting through EPO-Rs,165,193–196 although not all of the postulated effects of ESAs in this paradigm are deleterious.197 However, the immunohistochemical reagents utilized in these studies to demonstrate EPO-R have been shown to be nonspecific and to cross-react with heat shock proteins, which are known to be important in cancer.200–200 A more specific EPO-R antibody reagent has been developed,201 and functional EPO-R have not been detected on nonhematopoietic normal cells202 or on human tumor cell lines.203 Moreover, the tumor EPO-R hypothesis does not explain the observation that, within a given tumor type, the observed impact of ESA therapy on tumor outcomes is inconsistent and varies from study to study. The second mechanism proposed has been that ESA-induced changes in Hb levels change tumor oxygenation in ways that can be deleterious. Tumor cell hypoxia may affect tumor progression,32,35 and it is possible that tumor hypoxia occurs whenever Hb levels are substantially less or more than 12 to 13 g/dL.18 This theory might explain the variability in ESA effects on progression between trials within a given tumor type, but it does not explain the absence of observed deleterious effects in some trials in which ESAs were utilized at higher Hb levels178 or the safety signal derived from a trial in which Hb levels in the patients treated with ESA were actually moved toward the postulated optimum.204 Finally, it has been postulated that any negative effects of ESAs on tumor outcomes are due to the well-characterized impact of these agents on thrombosis risk.144 Because of the recently recognized possibility that anticoagulation may improve cancer survival207–207 and that an interplay exists between tumor progression and the coagulation system involved in tumor progression,210–210 this postulation is currently the most attractive hypothesis explaining any effects of ESAs on tumor progression or survival of patients with cancer.190 The finding that is of most concern regarding ESA effects on survival came from a very large trial in patients who had anemia and cancer and were not undergoing chemotherapy.204 The primary end point of this trial was transfusions, and no attempt was made to balance prognostic factors for survival between the two treatment arms. Baseline imbalances in prognostic factors favored the control arm; nevertheless, a lower survival rate was observed in the ESA-treated arm, with the observed effect in this study limited to males. Until further data become available regarding the safety and efficacy of ESAs in this patient population, ESAs should not be used routinely to treat anemia associated with cancer. Paraneoplastic polycythemia is an uncommon syndrome observed in a variety of human cancers,211 including renal cell carcinoma (associated with a von Hippel–Lindau mutation),214–214 hepatocellular cancers,215,216 Wilms tumor,217,218 and, rarely, other malignancies.219–222 The mechanism is usually ectopic production of EPO,219,220,223,224 although increased EPO levels are not always observed and other mechanisms, such as ectopic renin secretion, have been suggested. In renal cell carcinomas, in which inactivating mutations of the von Hippel–Lindau gene are common, accumulation of hypoxia-inducible factor, the transcription factor driving EPO gene expression, occurs frequently, sometimes causing polycythemia. In most cases of paraneoplastic polycythemia, Hb levels are only modestly elevated, presumably as a result of compensatory decreases in EPO production by the normal kidney. Rarely, polycythemia can be severe, with hematocrit levels exceeding 50% and/or the development of symptoms such as fatigue, headache, visual blurring, and dyspnea. In these cases, it is prudent to rule out other causes of polycythemia, including hypoxemia and coexisting myeloproliferative disorders, before treating the patient with phlebotomy or surgical removal of a tumor. By far the most common cause of neutropenia in oncology practice is the relatively straightforward myelosuppressive effects of cytotoxic chemotherapy and radiation treatment. Because of the mature neutrophil’s relatively short life span, neutrophil counts are particularly sensitive to the effects of recently administered chemotherapy, and nadirs of these counts are frequently observed 7 to 10 days after the administration of cytotoxic chemotherapeutic agents. Much less frequently, antibodies to neutrophils, bone marrow infiltration with disruption of normal marrow stromal function, and splenic sequestration can play a role. Neutropenia is a critically important problem in oncology practice for two reasons. First, neutropenia is the major factor driving the risk of life-threatening infections, which is one of the most serious and costly toxicities of cancer treatment.225,226 Second, neutropenia frequently results in substantial reductions in the delivered dose intensity of chemotherapy, causing even patients with potentially curable malignancies to receive less than the planned, optimal antitumor treatment. For both reasons, good neutropenia management is essential in oncology care. Although several glycoproteins have effects on neutrophil precursor cells, including interleukin-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage colony-stimulating factor, G-CSF appears to be the primary regulator of basal and emergency neutrophil production,227–232 as well as mature neutrophil function.235–235 GM-CSF plays a critical role in pulmonary homeostasis,236–239 and perturbations in this function are involved in the pathogenesis of pulmonary alveolar proteinosis.240–245 Negative regulatory factors of neutrophil production also exist that are less well understood, including neutrophil elastase,246 the src family kinases,247,248 and protein kinase C.249 Two effective strategies exist for the prevention of infection during myelosuppressive chemotherapy: the administration of myeloid growth factors and prophylactic antibiotics. Prophylactic antibiotics have the advantage of being less costly and the major disadvantage of selection of resistant bacteria, but the two strategies are not mutually exclusive. Three myeloid growth factor preparations are currently in use in clinical practice in the United States: recombinant G-CSF (filgrastim), pegylated recombinant G-CSF (pegfilgrastim), and recombinant GM-CSF (sargramostim). In randomized, controlled clinical trials in patients receiving myelosuppressive chemotherapy for nonmyeloid malignancy, filgrastim, administered as a daily subcutaneous injection at doses of 5 µg/kg, commencing the day after chemotherapy and continued until resolution of the white blood cell nadir (usually 10 to 12 days of treatment), has been consistently associated with a reduction in the duration of neutropenia and the incidence of febrile neutropenia across all cycles of chemotherapy.250–253 The results with sargramostim, usually administered at a daily subcutaneous dose of 250 µg/kg, have been less robust and less consistent, with some trials observing a reduction in febrile neutropenia across all planned cycles,256–256 other trials failing to demonstrate an impact on febrile neutropenia,257,258 and some trials demonstrating an effect on febrile neutropenia only during the first chemotherapy cycle.259,260 Some studies suggest that the myeloid growth factor can be started later during the chemotherapy cycle or can be given on less than a daily basis to conserve resources.261,262 However, in the best-powered randomized trial addressing the issue of late initiation of myeloid growth factor treatment that has been conducted, initiation of filgrastim treatment after neutropenia was established was not effective in reducing infection risk.263 Data suggest that a daily G-CSF dose of 2 µg/kg may be as effective as 5 µg/kg in shortening the duration of neutropenia after standard-dose chemotherapy.264 Pegfilgrastim has a longer half-life than filgrastim, particularly after the administration of chemotherapy.265 Because pegfilgrastim is cleared by neutrophils and their precursors, its half-life is prolonged by chemotherapy, and in this setting the drug is “self-regulating,” with levels persisting through the postchemotherapy nadir and until the neutrophil count recovers. In randomized, placebo-controlled trials in patients with nonmyeloid malignancies who were receiving myelosuppressive chemotherapy, pegfilgrastim given as a once-per-cycle subcutaneous dose on the day after the completion of chemotherapy was at least as effective as daily filgrastim in shortening the duration of neutropenia and reducing the incidence of febrile neutropenia.266–269 In these randomized, double-blind trials comparing pegfilgrastim to filgrastim, pegfilgrastim was not associated with more toxicity, and specifically, bone pain was not reported more frequently with pegfilgrastim. In a randomized, placebo-controlled trial involving patients with metastatic breast cancer, pegfilgrastim was associated with a reduction in the risk of febrile neutropenia.270 In early studies of myeloid growth factors, the incidence of febrile neutropenia in the control group was relatively high, approximately 40% or greater, and myeloid growth factor treatment was associated with a 50% reduction in this risk. In the placebo-controlled trial of pegfilgrastim, the incidence of febrile neutropenia in the control group was approximately 20%, and pegfilgrastim treatment was associated with a 95% reduction in risk. This demonstration of efficacy of myeloid growth factor therapy at lower risks of febrile neutropenia supports a change in practice, acknowledging the potential of myeloid growth factors to reduce lower risks of infection.271,272 The issue of the cost-effectiveness of the use of myeloid growth factors to prevent febrile neutropenia is controversial; the conclusion of the analysis depends in large part on assumptions regarding the cost of febrile neutropenic events (especially whether they are managed on an outpatient basis275–275) and the risk of febrile neutropenia in the patient population in question.226,276–281 Because use of pegfilgrastim is more convenient for patients, it has become the most frequently used myeloid growth factor for the reduction of infection risk during chemotherapy. The usual adult dose is 6 mg administered subcutaneously. Pegfilgrastim is both safe and effective when used with chemotherapy regimens that are administered every 2 weeks.282 When chemotherapy and myeloid growth factors are administered on the same day, it is possible that myeloid progenitors will be recruited into cell cycle while cytotoxic chemotherapy is still in their environment, with myeloid growth factors having the paradoxical effect of increasing myelosuppression.283 In practice, it remains prudent to administer myeloid growth factors, including pegfilgrastim, the day after the completion of chemotherapy. When deciding whether to administer myeloid growth factors during chemotherapy, it is appropriate for the clinician to assess the patient’s risk factors for infection, including the chemotherapy regimen being used, the patient’s functional status and comorbidities, age, and the presence of open wounds.284,285 If the risk of serious infection with the planned chemotherapy is unacceptably high, it is appropriate to use a myeloid growth factor, with or without prophylactic antibiotics, depending on the clinical setting. If the chemotherapy is being given every 2 weeks or less frequently, use of pegfilgrastim, at a dose of 6 mg administered on the day after the chemotherapy, is appropriate management. Myeloid growth factor therapy is associated with both an increase in neutrophil numbers and enhanced function of mature neutrophils.286 In animal models of sepsis, the addition of G-CSF to antibiotic treatment results in improved outcomes compared with antibiotics alone.287 It is therefore logical to investigate the combination of antibiotics and myeloid growth factors for the prevention of infection in patients undergoing chemotherapy who are at particularly high risk for infection. In a large randomized trial, the addition of filgrastim to prophylactic antibiotics (ciprofloxacin and roxithromycin) for patients undergoing chemotherapy for cancer was associated with a reduction in infection risk compared with antibiotics alone,288 although the authors raise questions regarding the cost-effectiveness of filgrastim in this setting.289 The clinician has two options in the management of a patient with cancer who is undergoing chemotherapy and is at risk of infection: prophylactic antibiotics290 and myeloid growth factors. For patients in whom the risk of infection remains unacceptably high despite prophylactic antibiotics, the addition of myeloid growth factor therapy will very likely further reduce risk. As noted previously, the initiation of myeloid growth factor treatment late in the chemotherapy cycle, after neutropenia has already occurred, may shorten the duration of neutropenia but is not associated with a meaningful reduction in the risk of infection. Several randomized trials of myeloid growth factors for the treatment of patients who are undergoing chemotherapy, have established febrile neutropenia, and have not been receiving prophylactic myeloid growth factor therapy for prevention of this complication have been conducted.291–296 Taken in aggregate, these studies document that treatment with either filgrastim or sargramostim probably shortens the duration of severe neutropenia, but for the typical adult patient with uncomplicated febrile neutropenia, this hematologic effect does not translate into significant clinical benefit in terms of reduction in the duration of hospitalization or parenteral antibiotic use. For the exceptional patient who is quite ill and in whom a modest reduction in the duration of neutropenia may be expected to be of benefit, the initiation of myeloid growth factor treatment is prudent.297 For these patients, either filgrastim at a dose of 5 to 10 µg/kg per day or sargramostim, 250 to 500 µg/m2 per day, is a reasonable treatment approach. When chemotherapy is being given with the intention to cure or significantly prolong life, substantial dose reductions may compromise those therapeutic goals. Studies of records from community oncology practices suggest that the administered dose intensity of both adjuvant breast cancer chemotherapy and lymphoma treatment are frequently substantially lower than the published and planned regimens, suggesting that chemotherapy dose reductions and delays are common, even when cure is the therapeutic goal.298,299 In these and other studies, myeloid growth factor treatment use was highly variable between practitioners, and these agents were usually not used to maintain dose intensity. Myeloid growth factors can be used to enhance the delivered dose intensity and support the administration of full chemotherapy doses on time in these settings.302–302 In clinical practice, when good evidence indicates that a given chemotherapy regimen administered in full, planned doses given on time produces an improvement in cure rate or survival, it is obviously prudent to use myeloid growth factors rather than dose delays or reductions to manage bone marrow tolerance and infection risk. In patients with cancer who do not have an infection, leukocytosis occurs in oncology practice in conjunction with myeloid growth factor treatment as a result of marrow involvement with tumor with a leukoerythroblastic pattern in the peripheral blood smear, or, rarely, as a paraneoplastic syndrome. Occasionally, cancers will produce ectopic G-CSF or GM-CSF, resulting in a leukocytosis with a differential pattern reflective of the specific ectopic cytokine.303–306 In patients with squamous cell carcinomas, a paraneoplastic leukocytosis with hypercalcemia with or without cachexia and thrombocytosis can occur.307–311 The pathophysiology of this syndrome is usually production of parathyroid hormone–like peptides coupled with G-CSF and, occasionally, other cytokines.306,312–317 In general, paraneoplastic leukocytosis does not require specific therapy; knowledge of its existence is primarily important in aiding the clinician in differential diagnosis. When a patient with cancer presents with an unexplained leukocytosis, it is prudent to obtain a serum calcium level and consider antiresorptive therapy if the level is elevated. The primary regulator of the platelet count in humans is TPO,318,319 a glycoprotein that is produced in the liver and is cleared by platelets and their precursors. TPO induces growth and development of megakaryocytes320; assuming stable liver function, levels fluctuate with changes in platelet count because of variations in clearance of the molecule. In mammals, treatment with interleukin-11 (IL-11) induces a modest increase in platelet counts, but IL-11 is not required for thrombopoiesis321–324; its primary constitutive role seems to be the maintenance of female fertility.325 Thrombocytopenia that is encountered in oncology practice may be due to the effects of chemotherapy, particularly with agents such as bortezomib, gemcitabine, carboplatin, or ifosfamide, or after multiple cycles of treatment. It is also encountered in patients with cancer who have liver disease (due to decreased TPO production), immune thrombocytopenia (ITP), particularly in patients with lymphoid malignancies or infection with the human immunodeficiency virus, disseminated intravascular coagulation associated with adenocarcinomas or infection, therapy-related thrombotic thrombocytopenic purpura, and drug-induced thrombocytopenia, as well as in patients with splenic sequestration. Occasionally patients with underlying collagen vascular diseases present with amegakaryocytic thrombocytopenia as a result of autoantibodies directed against the TPO receptor (TPO-R).326,327 Recombinant IL-11, oprelvekin, has been shown to accelerate platelet recovery after chemotherapy and to reduce platelet transfusion burden in transfusion-dependent patients with chemotherapy-associated thrombocytopenia.330–330 Oprelvekin is approved by the FDA for this indication. However, toxicities of this agent are substantial and include fluid shifts, cardiac arrhythmias, optic neuropathy, and the potential for anaphylaxis; these toxicities have limited the usefulness of this drug in oncology practice. Oprelvekin is administered at a dose of 50 µg/kg per day, as a daily subcutaneous injection, commencing the day after chemotherapy and continuing until the nadir has past and the platelet count has returned to 50,000 cells/µL; the drug should be stopped 2 days before the next chemotherapy dose is given. For patients with significant renal impairment, the daily dose is reduced to 25 µg/kg per day. The cloning of human TPO was met with hope that this agent would represent a safer platelet growth factor for clinical oncology practice. Both a full-length clone of recombinant human TPO (rTPO) and a truncated, pegylated preparation of this molecule, megakaryocyte growth and differentiation factor (MGDF),331,332 were developed and introduced into clinical trials. Therapy with either rTPO333–339 or MGDF331,332,340–345 was associated with an increase in the number of circulating, functional platelets and platelet progenitor cells and with a reduction in the duration of postchemotherapy thrombocytopenia. No clear difference between the safety and efficacy of the two preparations was found, and neither was associated with fluid shifts or arrhythmias. Unfortunately, in some patients treated with MGDF, antibodies to TPO developed,346 resulting in sustained thrombocytopenia due to cross-reacting antibodies, and the development of this molecule was discontinued in the United States. For reasons that are less clear, the development of rTPO was not completed and therefore neither agent is available for prescription in the United States. However, the potential of a nonimmunogenic TPO-R agonist to provide oncologists with a rational, safe, and effective alternative to oprelvekin has been demonstrated. By the time these new TPO-R agonists entered clinical development, it was clear that ITP was associated with a relative TPO deficiency and that the thrombocytopenia in that setting would improve with treatment with a TPO-R agonist.349–349 The relative deficiency occurs because the megakaryocyte mass is the primary best predictor of TPO clearance, and an expanded megakaryocyte pool is associated with decreased TPO levels.352–352 Both romiplostim353–358 and eltrombopag359–362 have been found to be effective in the treatment of ITP, and both drugs are approved for this application by the FDA. These agents are also under development for the treatment of thrombocytopenia associated with liver disease,363 myelodysplasia,366–366 congenital mutations,367 and chemotherapy.368 Eltrombopag does not activate mature platelets, which may reduce any thrombosis risk associated with TPO therapy.362,369 Both of these agents are useful for oncologists who need to treat patients with ITP, but the agents are not yet developed for the treatment of thrombocytopenia associated with chemotherapy. When thrombocytosis is encountered in oncology practice, it is most often due to functional or absolute iron deficiency, infection or inflammation, or hyposplenism. As noted previously, thrombocytosis can also occur in conjunction with leukocytosis and hypercalcemia as a paraneoplastic syndrome, usually occurring in patients with a squamous cell malignancy. Less commonly, it can occur as an isolated paraneoplastic syndrome associated with hepatocellular carcinoma370 (presumably due to ectopic TPO) or in association with ovarian cancer, where the thrombocytosis appears to be driven by tumor production of IL-6 and is associated with a poor prognosis thought to be due to a paracrine loop.371 Obviously, thrombocytosis can also result from a coexisting myeloproliferative disorder such as essential thrombocytosis or polycythemia vera. It is important to exclude myeloproliferative disorders, for which antiplatelet prophylaxis may be necessary. In most other instances, the thrombocytosis does not require specific intervention. Myelodysplastic syndrome (MDS) is quite common in oncology practice and usually presents as anemia, with or without thrombocytopenia or neutropenia. The reader is referred to Chapter 99 for a complete discussion of this topic. It is important to consider myelodysplasia in the differential diagnosis of anemia, thrombocytopenia, or leukopenia, especially in patients who are elderly or have been treated in the past with cytotoxic chemotherapy. For the anemia that occurs in these patients, ESAs have been shown to increase Hb levels and/or reduce transfusion requirements in 30% to 60% of patients with low or intermediate-1 stage disease.372–389 Some evidence indicates that coadministration of a myeloid growth factor may enhance the erythropoietic response to ESAs in this setting and enhance survival,390–398 although the cost-effectiveness of this approach has been questioned.399,400 It is reasonable to treat a patient with either transfusion-dependent or symptomatic anemia and international prognosis scoring system low or intermediate-1 stage MDS with an ESP alone or an ESP with a myeloid growth factor and to continue this therapy if it is effective in improving clinical status and is not associated with increasing thrombocytopenia or the percentage of circulating blasts. In early clinical trials, myeloid growth factors were shown to increase the neutrophil counts401–409 and improve neutrophil function409–409 in neutropenic patients with MDS. Myeloid growth factors have also been used to support myelosuppressive therapy for MDS.410,411 Currently, the data do not support routine long-term administration of myeloid growth factors to patients with MDS, except to support the treatment of anemia. Short-term administration of myeloid growth factors to support myelosuppressive therapy or to transiently increase neutrophil counts or function during an infection episode is reasonable. In patients with acute nonlymphocytic leukemia (acute myeloid leukemia [AML]), prolonged and profound cytopenias develop during induction and consolidation chemotherapy. The reader is referred to Chapter 98
Disorders of Blood Cell Production in Clinical Oncology
Introduction
Disorders of Red Cells
Anemia
Pathophysiology
Management
Increasing Importance of Iron and Iron Metabolism
Preparation
Brand Name
Administration
Low-molecular-weight dextran
INFeD
Anaphylactic reactions have been reported, and an intravenous test dose of 0.5 mL infused over ≥30 sec is recommended before the first dose; intravenous doses containing ≤100 mg elemental iron (2 mL) can be given at a rate of ≤50 mg (1 mL) per minute as frequently as daily; TDI calculated as dose (mL) = 0.0442 (desired Hb − observed Hb) × LBW + (0.26 × LBW) is feasible in one session*; premedication with corticosteroids will decrease the frequency of myalgia and arthralgias after TDI†
High-molecular-weight dextran
Dexferrum
Similar to low-molecular-weight dextran, although reported rates of adverse drug reactions are greater,60,61 and the use of this preparation with ESP therapy in patients with cancer cannot be supported
Ferric gluconate complex
Ferrlecit
A test dose is not required; TDI is not feasible because of a high frequency of adverse events when doses of >10 mL (125 mg iron) are given in a single session; doses of up to 125 mg can be diluted in 100 mL of normal saline solution and infused intravenously over 1 hour, or the solution can be pushed undiluted at a rate of 1 mL (12.5 mg) per min
Iron sucrose
Venofer
A test dose is not required; TDI is not feasible, although doses of up to 400 mg can be administered by slow infusion over 3 hours; doses of 100-200 mg can be given by slow intravenous push over 5 min; alternatively, 100 mg can be diluted in 100 mL of normal saline solution and infused intravenously over 15 min or more
Safety of Erythropoiesis-Stimulating Agents
Polycythemia
Disorders of White Cells
Neutropenia
Pathophysiology
Management
Prevention of Infection
Treatment of Established Neutropenia or Neutropenic Infection
Use of Myeloid Growth Factors to Maintain Chemotherapy Dose Intensity
Leukocytosis
Disorders of Platelets
Thrombocytopenia
Pathophysiology
Management
Thrombocytosis
Acquired Marrow Failure States
Myelodysplastic Syndrome
Acute Nonlymphocytic Leukemia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Disorders of Blood Cell Production in Clinical Oncology