Test
Cutoff
Sensitivity (%)
Specificity (%)
OR (95%CI)
aPL score
APTT mixing confirmation test, ratio
>49s
39.1
89.3
5.36(2.53–11.4
5
>1.3
19.6
95.2
4.81(1.79–12.9)
2
>1.1
30.4
90.9
4.38(1.96–9.76)
1
KCT mixing
>29s
45.6
88.8
6.64(3.17–13.9)
8
dRRVT mixing confirmation test, ratio
>45s
28.2
90.9
3.93(1.74–8.88)
4
>1.3
17.4
94.7
3.72(1.38–10.1)
2
>1.1
28.3
90.4
3.7(1.65–8.27)
1
IgG aCL, GPL high titers
>30
15.2
98.4
11(2.72–44.5)
20
IgG aCL, GPL medium/low titers
>18.5
19.5
94.6
4.31(1.63–11.3)
4
IgM aCL, MPL
>7
6.5
96.3
1.79(0.45–7.22)
2
IgG aβ2GPI, units high titers
>15
23.9
98.4
19.3(5.11–72.7)
20
IgG aβ2GPI, units medium/low titers
>2.2
30.4
92.5
5.4(2.35–12.4)
6
IgM aβ2GPI, units
>6
8.7
91.4
1.02(0.32–3.20)
1
IgG aPS/PT, units high titers
>10
19.6
97.8
11.1(3.25–38.1)
20
IgG aPS/PT, units medium/low titers
>2
28.3
95.7
8.81(3.39–22.9)
13
IgM aPS/PT, units
>9.2
6.5
98.9
6.45(1.05–39.8)
8
The prevalence of APS manifestations in patients with aPL-S = 0 was 10%, for those with 1–9, 26%; for those with 10–29, 29%; and for those with >30, 56%. Similar results were observed with the partial aPL-S. Receiver-operating characteristic [23] curves for APS diagnosis using aPL-S, partial aPL-S, and revised Sapporo criteria all showed a hyperbolic pattern, implying that aPL-S is a potential quantitative marker for APS diagnosis.
Regarding predictive ability , the aPL-S and partial aPL-S were higher among patients in whom thrombosis developed than among those without thrombosis (median score 5.5 in aPL-S with thrombosis vs. 0 and 5.5 vs. 0 in partial aPL-S). Odds ratios (OR) for new thrombosis in patients with aPL-S of ≥10 and ≥30 were 2.9 and 5.3, the positive predictive values 20% and 31%, and the negative predictive values 92% and 92%.
In a multivariate Cox regression tests that included age, gender, glucocorticoid treatment, hypertension, hyperlipidemia, diabetes, SLE, or rheumatoid arthritis, an aPL-S of ≥30 was an independent risk factor for thrombosis (OR 3.144 [95% CI 1.383–7.150], p < 0.006) [23].
Global Antiphospholipid Syndrome Score (GAPSS)
The global APS score (GAPSS) (Table 9.2) was first developed and validated in a large cohort of patients with SLE, divided into two statistically independent sets by a computer-generated randomized list [24]. According to this model, risk assessment quantification was based on the computation of independent factors for thrombosis and pregnancy loss. The variables identified by the multivariate analysis to be independently related to thrombosis or pregnancy morbidity include criteria (LA, aCL, and aβ2GPI) [1] and non-criteria (antiprothrombin antibody, aPS/PT) aPL [25] and cardiovascular risk factors of arterial hypertension and hyperlipidemia. Global APS score gives these factors weights proportional to the β-regression coefficient values: five points for IgG/IgM aCL, four for IgG/IgM aβ2GPI and LA, three for IgG/IgM aPS/PT and hyperlipidemia, and one for arterial hypertension. In a prospective assessment of 51 SLE patients followed for a mean 34 months, an increase of >3 GAPSS points the best predicted risk for vascular events (OR 48 [95% CI 6.90–333.85, p = 0.0001]) [26]. In 62 consecutive patients with PAPS, higher GAPSS values occurred in patients with thrombosis than with pregnancy morbidity [4]. Patients with recurrent thrombosis had higher GAPSS than did those without recurrences. Patients with GAPSS ≥11 points had an 18-fold increase in risk of recurrence, conclusions confirmed by Oku et al. [5] in a retrospective cohort of 41 APS and 241 control patients. Higher GAPSS values occurred in patients who had experienced arterial and/or venous thrombosis. Zuily et al. evaluated the power of GAPPS to predict thrombosis in a prospective multicenter cohort study [6]. Of 137 consecutive patients with aPL or SLE (mean age 43.5) followed for a mean of 43 months, patients who experienced thrombosis had a mean GAPSS score of 10.9 compared to those with no thrombosis (GAPSS 8.2). In multivariate analysis, GAPSS >16 was the only predictor of thrombosis (OR 6.2 (95% CI 1.70–22.40)).
Table 9.2
The global antiphospholipid syndrome score (GAPSS)
Factor | Point value |
|---|---|
Anticardiolipin IgG/IgM | 5 |
Anti-β2-glycoprotein-I IgG/IgM | 4 |
Lupus anticoagulant | 4 |
Antiphosphatidylserine/prothrombin (aPS/PT) IgG/IgM | 3 |
Hyperlipidemia | 3 |
Arterial hypertension | 1 |
Antiphospholipid Antibody-Related Damage Measurement
In an early study that addressed functional outcomes after 10 years, Erkan et al. reported that 38% of PAPS patients had reduced functional status as a result of hemiparesis, dementia, quadriplegia, or cardiac compromise [27]. Shah et al. reported that 9 of 31 (29%) patients with APS had further thromboses during 10 years’ follow-up [28]. A systematic review based on a multicenter European survey reported increased mortality rates (5.3–6.7%) of APS patients, primarily due to arterial thrombosis [29]. Quality of life (QoL) issues related to obstetrical complications are also important to long-term prognosis [30].
The SLICC/ACR damage index (SDI) measures cumulative organ damage but lacks specificity for APS. Grika et al. [31] as well as Barbhaiya et al. [32] analyzed the usefulness of SDI in patients with aPL, APS, SLE, and other autoimmune conditions. They found that SDI increases with damage but were unable to identify organ damage directly related to aPL, suggesting that SDI overestimates damage related to lupus and minimizes that related to aPL.
A damage index in APS (DIAPS) has been designed to include APS-specific items , all thrombotic, not considered in SDI [7]. DIAPS has solid methodological steps [33]. It measures irreversible damage in different systems affected by APS. DIAPS consists of 37 items and can be completed by an untrained physician in 20 min. The construction and validation of the questionnaire are shown in Fig. 9.1. A Delphi panel performed with an international group of experts showed a high reliability in 94% of its items.
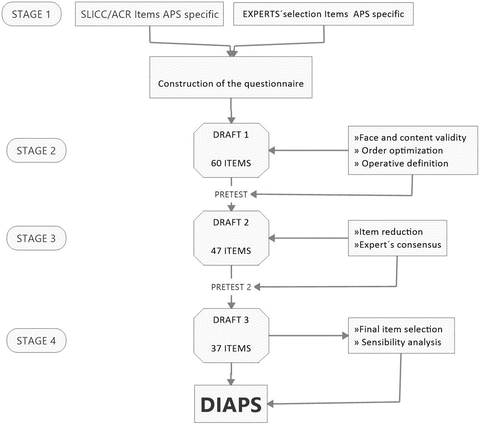

Fig. 9.1
Damage index in antiphospholipid syndrome (DIAPS) development and validation
DIAPS is the first published report of the development and validation of a questionnaire to assess aPL-related organ damage in patients with APS [7]. In its initial validation, DIAPS was applied to 156 patients with thrombotic APS from four Latin American countries. A key step was the simultaneous application of a generic quality of life instrument, EuroQoL. DIAPS had a high internal consistency. The EuroQol domains of mobility, pain/discomfort, health status, usual activities, and self-care correlate with the global DIAPS score. Ugolini Lopes et al. [34] compared SDI and DIAPS in 93 PAPS patients and found correlation between duration of illness and higher scores with both indices; DIAPS seemed to be a better measure of severity in APS.
Non-thrombotic manifestations of the syndrome such as livedo reticularis/racemosa, multiple sclerosis-like CNS disease, and diffuse pulmonary hemorrhage were not included in DIAPS, although “non-criteria” manifestations are part of the wide spectrum of APS [35] and many common manifestations of APS are not thrombotic. The impact that obstetric morbidity of APS and aPL have on chronic damage remains unknown.
Suggestions regarding items that may improve the reliability and accuracy of DIAPS include need of an operational definition of pulmonary hypertension and neuropsychiatric manifestations (such as cognitive dysfunction), and of adding damage secondary to bleeding from excessive anticoagulation [36], alveolar hemorrhage, adrenal hemorrhage, severe thrombocytopenia, hypoprothrombinemia, and catastrophic APS. Further, because DIAPS items are binary, weighing each item may give DIAPS more clinical relevance . Future research requires long-term follow-up of the patients to establish sensitivity, specificity, predictive values, sensitivity to change, and reliability. DIAPS was developed and validated using specific aPL manifestations; other manifestations should be added. Because DIAPS was developed based on Latin American patients, further studies should include additional populations.
Antiphospholipid Antibody-Related Quality of Life Measurement
Quality of life includes health as well as other domains, such as jobs, schools, culture, and values; it comprises both physical and mental health. Health-related quality of life (HRQoL) predicts mortality and morbidity [37] and is impaired in patients with history of venous thrombosis [38] or of SLE [39]; SLE-related disease activity and damage over time predict poor HRQoL [40]; recent studies ask whether APS or aPL impair HRQoL [41].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree